A Visual Link to Human and Veterinary Medicine
At the Texas A&M College of Veterinary Medicine & Biomedical Sciences (CVM), modern medical imaging technology and techniques have transformed how researchers and doctors view and interpret cells and tissues. The CVM has assembled an unparalleled collection of advanced imaging technologies that are being used in basic, clinical, and translational research as well as diagnostic imaging and therapeutic intervention. The high resolution images generated from these technologies range from single molecules at the nanometer-size scale to whole organ functional imaging on the meter scale. These images, in turn, have advanced research and treatment of a number of diseases and conditions, especially cancer.
Imaging History

Microscopy started with two pieces of glass in a Dutch spectacle-maker’s workshop in the 1590s. Hans Jansen and his son, Zacharias, experimented with lenses in a tube and found their invention created a magnified image of any object viewed through it. The invention, a compound microscope, was used by Robert Hooke to view and draw various life specimens for his book, “Micrographia,” published in 1665. Inspired by Hooke’s drawings and observations, Anton van Leeuwenhoek, later known as the father of Microbiology, improved the microscope to pursue his own studies. His improvements allowed him to be the first person to see and write about single-celled organisms, blood vessels, bacteria, and other microscopic biological entities.
Innovation in microscopy technology stalled for two centuries. Then, in the 1850s, Carl Zeiss, an engineer who manufactured microscopes, began tweaking the lenses. He employed glass specialist Otto Schott to improve the lens quality and Ernst Abbe to refine the manufacturing process. The collaboration of the three men produced the modern compound microscope found in labs and classrooms around the world today.
Other forms of viewing patients and their biological samples were developed later. X-rays were discovered in 1895, and contrast agents followed a decade later. By the 1950s, radiation technology had been sufficiently developed for the initial uses of nuclear medicine to begin. Computed tomography (CT) and magnetic resonance imaging (MRI) techniques became available in the 1970s.
Over time, these innovative technologies have revolutionized how physicians and researchers are able to study, diagnose, and treat conditions. In the modern era, all of these medical imaging modalities and more have found a place at the CVM and continue to improve medical science and research.
Image Analysis Laboratory

The Image Analysis Laboratory (IAL) began as an electron microscope (EM) facility in 1987. EM continues to be an important tool for research and diagnostics. This aspect of the laboratory is managed by Dr. Ross Payne, associate research scientist in veterinary pathobiology, who provides a wide range of EM techniques, data analysis, and training for CVM scientists.
The first confocal microscope joined the ranks in 1990. “Around this time, there was a renaissance in light microscopy technology due to the integration of laser light sources, computers, and sensitive camera systems with microscopes along, with the new field of biophotonics,” said Dr. Robert Burghardt, director of the IAL and associate dean for research and graduate studies.
The development of biophotonics, a set of optical techniques for studying biological samples, gave the lab even more ways to examine cells.
“This technology, when it was integrated, allowed us to ask new questions and look into cells in a noninvasive way to eavesdrop on a variety of different functions, cell behaviors, and basic homeostatic mechanisms,” said Burghardt. “We could ask questions about how cells respond to an incredible number of environmental factors, such as exposure to hormones, growth factors, mechanical forces, and environmental chemicals.”
With these advancements in the available technology, Burghardt realized having someone with an engineering background would bring much needed scientific knowledge and mathematics expertise and a fresh viewpoint to IAL. He hired Dr. Roula Mouneimne, associate director of IAL, in 1990 to bring those attributes to the lab.
“He had a vision,” Mouneimne said of Burghardt hiring her. “In 1990, not many people were looking to integrate engineering principles in biological applications.”
“With her expertise as an engineer, she can help investigators from many disciplines to integrate the acquisition and processing of the data with high-end computational methods and statistical approaches,” Burghardt said.
IAL grew further. The lab now supports the Texas A&M System as an Advanced Imaging Core Facility for the Center for Translational Environmental Health Research (CTEHR). Funding for the CTEHR is provided by the National Institute of Environmental Health Science (NIEHS). In this role, the core supports the center’s goal to “improve human environmental health by integrating advances in basic, biomedical, and engineering research across translational boundaries from the laboratory to the clinic and to the community and back.” In addition, IAL acts as a core for the Center for Organ and Cell Biotechnology, a joint effort of the Texas Heart Institute (THI) and the CVM that seeks to create and eventually market disruptive cell and organ biotechnologies and molecular tools for the next generation of medicine.
“We also provide services to a large cross-section of the campus,” said Burghardt. “Our lab has been a core facility for interdisciplinary grants. There have been people working on reproductive biology, toxicology, biochemistry, neuroscience, chemical biology, cell signaling, and cancer biology. We support scientists with experimental design, data collection, and analysis that lead to new knowledge in their particular disciplines. Acting in so many capacities requires the laboratory to have adapted to the many interdisciplinary needs of the groups that utilize it. The lab’s imaging capabilities have grown from electron microscopy to noninvasive live-cell imaging tools that can visualize processes at the tissue and cell level to the single molecule level. The wide range of microscopic technologies acquired by the lab are available to researchers allowing them visualize cells in greater detail and investigate new research areas.
“Over the last 30 years, we have been able to ask more complex questions,” said Burghardt. “Neuroscientists, reproductive biologists, toxicologists, and scientists from other disciplines of modern biology are realizing that functional imaging is a way to understand basic biological phenomena and processes.”
One example of a major imaging technique used in IAL is multiphoton fluorescence microscopy. With the application of a special laser, select molecules can be illuminated. Based on the spectral pattern fluoresced by the sample, one class of common lipid soluble carcinogenic molecules, produced in grilled food and other products of combustion, can be identified as they are forming in living cells. Identification of these molecules has led to development of treatments that eliminate the formation of these carcinogenic molecules. Besides cellular imaging research support, graduate training in the theory and practice of optical microscopy technologies is a major emphasis of the IAL.
For example, students are trained to use different microscopes while applying techniques such as co-localization of two molecules or transient protein-protein interactions and image processing. Students learn how to apply computational approaches to determine with confidence the outcomes of their experiments. Workshops and individual consultations provide additional avenues for students and faculty members to utilize the expertise of the lab as well as its tools.
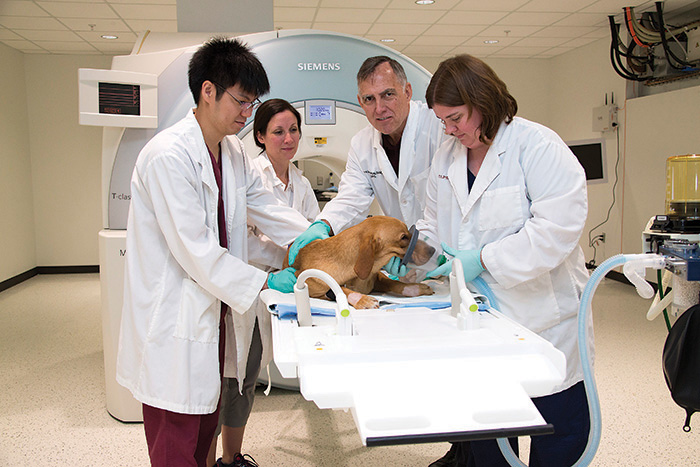
Diagnostic Imaging & Cancer Treatment Center

The Diagnostic Imaging & Cancer Treatment Center (DICTC) was formed in 2011 when the CVM gained a 3-Tesla MRI. This powerful tool allowed researchers and clinicians at the CVM to quickly capture detailed images of tissues and cells in small and large animals.
“When the MRI was commissioned for clinical use, it was one of three in all of Texas. That includes human facilities,” said Dr. Michael Deveau, clinical assistant professor in oncology. “We have a veterinary facility with technology that most of the state of Texas didn’t have access to at the time, including human patients and clinicians.”
The center’s capabilities are not limited to MRI. Other imaging modalities include small and large animal radiology, small animal ultrasound, CT scans, and nuclear medicine. Utilizing multiple types of imaging technology improves the ability of the clinicians to provide an accurate diagnosis and create a treatment plan.
“The literature supports no single modality as superior to any of the others,” Deveau said of the various imaging options available at the center. “They all have their strengths and weaknesses. When you put the information that you acquire from all of them together, that’s when you see a tremendous benefit, as compared to any single modality by itself.”
DICTC has two primary goals. First, it aims to advance veterinary healthcare by providing options and solutions for conditions that animal patients face. Second, it fulfills part of the One Health Initiative.
“The facility was built in part to answer the Initiative,” said Deveau. “It was a huge intellectual and financial investment by Texas A&M University, the CVM, and the donors. The facility helps utilize veterinary companion animals as representative translational models for human conditions.” Both goals are broad and cover a wide range of fields and research studies.
“I think imaging is quite a large topic. It has its fingers in practically every aspect in medicine,” said Deveau of the breadth of the center’s abilities.
One example Deveau gave of how the center’s equipment can be applied involved creating a 3-D model of a patient’s gut. The virtual model, constructed from CT scans of the patient’s digestive system, can be used to understand how a piece of food travels through and is processed by the patient’s gut. Such a model, of any system or organ or even a section of tissue, can have multiple applications for both research and clinical practice.
Additional imaging technologies can also be utilized for the same patient to add greater depth to the diagnostic picture, but at a cost. In addition to the financial expense associated with testing, animal patients also have special considerations, as many types of imaging require the animal to be under general anesthesia to ensure they remain motionless.
“When you start talking about doing multi-modality imaging, it adds up quickly,” explained Deveau. “Even if you combine it all under one round of anesthesia, you still have the cost that comes out of pocket for the tests and the anesthesia.”
Still, being able to use multiple tests to develop a comprehensive understanding of a patient’s unique needs can be invaluable for conditions such as cancer. Precise imaging is particularly important when using targeted radiation therapy, as it is essential to know exactly where the cancer cells are before you can attack them. In addition to revealing location, imaging also allows doctors to monitor tumors during therapy. Being aware of changes in a tumor’s size or the presence of additional tumors can help doctors know if a therapy is working or if it needs to be modified.
“The more modalities you use, the more information you get,” said Deveau. “Having more information will potentially set you up for a more optimal therapy.”
Texas A&M Institute for Preclinical Studies
Just as the IAL supports researchers who apply imaging techniques at the cellular and subcellular level, the Texas A&M Institute for Preclinical Studies (TIPS) performs imaging studies on whole animals involved in research projects. Established by the Texas A&M Board of Regents in 2007 and opened in 2009, TIPS is an administrative unit of the CVM. Research at TIPS is done in collaboration with Texas A&M faculty and investigators from private companies who wish to establish efficacy of new drugs or medical devices before moving them to human use.
From its inception, TIPS has heavily emphasized biomedical imaging. Indeed, imaging methods and equipment at TIPS-extending from conventional radiography to a 3-Tesla MRI machine-largely parallel those used through the DICTC. Dr. Joe Kornegay, TIPS director, said, “The availability of similar instrumentation in the hospital and at TIPS provides a remarkable opportunity for collaboration, whereby studies done in each unit can inform and complement the other.” One such example is a specialized imaging technique at TIPS called positron emission tomography–computed tomography (PET-CT). This combines the anatomical detail gained by CT with information on organ function provided by PET scans. Through a collaboration involving Deveau and a veterinary oncologist, Dr. Heather Wilson-Robles, TIPS is conducting PET-CT scans on animal cancer patients to determine the extent of tumor metastasis. This allows oncologists to better plan treatment for the affected animal and, at the same time, give owners a more accurate prognosis.
Kornegay has used specialized imaging in his own research involving a canine model of Duchenne muscular dystrophy. In fact, when he relocated to Texas A&M; from the University of North Carolina–Chapel Hill three years ago, Kornegay immediately started working with the TIPS imaging group to conduct MRIs on dystrophic dogs involved in research studies. A veterinary neurologist by training, Kornegay began using CT and MRI in the 1980s to diagnose disease in animal patients while on the faculty at North Carolina State University. These initial studies were done at Duke University Medical Center before CT and MRI were widely available in veterinary schools.
“It’s not an overstatement to say that sophisticated imaging modalities have truly revolutionized medicine,” Kornegay stressed. “For the diagnosis of brain disease, much of the guesswork inherent to other imaging methods is removed by the anatomical detail provided first by CT and later by MRI.” Kornegay sees many of the same advantages when these techniques are applied in a research setting. “By definition, imaging is largely noninvasive and, beyond the ‘pretty pictures’ themselves, provides quantitative data that can be collected at multiple time points and compared statistically,” he said. “This is extremely powerful from a research perspective.”
The Future
We’ve come a long way since Robert Hooke peered through an early version of the microscope and observed tiny organisms in detail previously unavailable to scientific inquiry. How imaging will continue to evolve and adapt is uncertain, but it will continue to affect how we view, perceive, and respond to conditions not visible to the naked eye.
“Imaging serves different purposes depending on whom you speak to,” said Deveau. “I think the biggest thing from a discovery perspective is that, with the level of technology we have, it is bringing to life different changes in the information that we get from imaging, and that dictates or directs how we manage the patient.”


