Confused about Lipase Assays?
- During pancreatitis, pancreatic lipase is released into the bloodstream and can be used as a diagnostic marker for the disease.
- Other lipases are released from various other organs, adding to the total serum lipase activity.
- PLI assays (Spec cPL® and Spec fPL®) specifically measure lipases of pancreatic origin, making them very sensitive and specific for a diagnosis of pancreatitis.
- DGGR- and triolein based lipase assays measure the total serum lipase activity and thus are unspecific.
Conclusion
- The measurement of PLI concentration by Spec cPL® in dogs and by Spec fPL® in cats remains superior for the diagnosis of pancreatitis in both dogs and cats.
Acute or chronic pancreatitis are now recognized to be common in both dogs and cats, but their diagnosis can be challenging. Various diagnostic tests have been used to confirm a diagnosis of acute and chronic pancreatitis, including the measurement of serum lipase activity, measurement of serum pancreatic lipase immunoreactivity (PLI), abdominal ultrasound, and histopathology.
Assays for the measurement of serum lipase activity were commonly used in the past because they were minimally invasive, inexpensive, and did not require special equipment or expertise. Although the exocrine pancreas is a major source of the serum total lipase activity, it is not the only organ or type of cell that produces, stores, and releases a lipase. Lipase may be released by the stomach (gastric lipase), the liver (hepatic lipase), endothelium (endothelial lipase), and by many other organs and cells. All of these lipases may to some degree contribute to the total lipase activity measured in serum. Thus, assays for the measurement of serum total lipase activity lack specificity for pancreatic lipase. In addition, they are not specifically sensitive for the diagnosis of pancreatitis. Depending on the cutoff value used, total serum lipase activity has been reported to have a sensitivity as low as 13.6% for macroscopic pancreatitis, thus missing up to 86.4% of patients with pancreatitis.1 The sensitivity and specificity naturally change with different cut-off values. This explains why some studies found higher sensitivities (up to 71%) but at the expense of specificity with an increasing number of false-positive results (specificity 43%, i.e. 57% of positive results are false-positive).2 Furthermore, renal,3,4 gastrointestinal,5 hepatic, and neoplastic disease,6 as well as steroid administration7 have all been shown to cause increases in total serum lipase activity.
Serum pancreatic-lipase immunoreactivity (PLI) as measured by the Spec cPL® or Spec fPL® specifically measures lipase that originates from the acinar cells of the canine or feline pancreas, respectively. These assays use antibodies directed against pancreatic lipase and to date, no other lipase has been shown to cross-react with these antibodies.8-10 Therefore, Spec cPL® and fPL® are considered to be the most specific diagnostic tests for the exocrine pancreas. Also, the measurement of Spec PL® is highly sensitive for a diagnosis of pancreatitis in both dogs and cats. In dogs with clinically significant signs of pancreatitis, Spec cPL® has been shown to identify the disease with a sensitivity of 82 to 94%. In dogs with less severe pancreatitis, Spec cPL® still showed the highest accuracy among any diagnostic test with a sensitivity of 64%.1 In cats, Spec fPL® correctly identified patients with pancreatitis with a sensitivity between 54% (subclinical to mild disease) to 100% (moderate to severe disease). Specificities for Spec cPL® and fPL® are even higher and have been reported to be between 79 and 100%. Thus, these tests rarely produce false-positive results. This is especially important as many other diseases may cause clinical signs similar to those seen with pancreatitis.
More recently, a semi-quantitative assay (VetScan cPL rapid test for VetScan VUE, Abaxis) has been introduced for use in dogs only. While no analytical or clinical validation data has been published so far, the manufacturer claims that the assay will determine the cPLI concentration within a window of +/- 60 µg/L. A limited validation study, however, showed poor linearity, accuracy, and reproducibility of the assay, suggesting that it would not be valuable for clinical evaluation of patients with suspected pancreatitis. For example, one sample with a Spec cPL of 566 µg/L had two results within the reference interval (109 and 152 µg/L), 4 within the questionable range (247, 270, 297, and 392 µg/L), and 2 in the diagnostic range for pancreatitis (467 and 477 µg/L). Two other assays (manufactured by Bionote and Samsung) have more recently been introduced into the marketplace, but no validation or performance data are available as of yet.
Recently, two substrates for the measurement of total serum lipase activity (triolein and 1,2-o-dilauryl-rac-glycero-3-glutaric acid (-6’-methylresorufin, DGGR)) have been marketed for the measurement of total serum lipase activity. Both substrates are used in catalytic assays, where serum lipases cleave the substrate and products of this reaction are measured via colorimetry. The v-LIP-P slide detects serum lipases using triolein as the substrate and a negatively charged detergent as an auxiliary agent11 while the DGGR-lipase assays use DGGR as a substrate.12 Both substrates have been claimed to have a comparable diagnostic utility to that of serum Spec PL.
In 2005, Graca et al. validated a DGGR lipase assay for use in dogs.13 Their study showed, that using a cut-off value of 120 U/L, this test had a high sensitivity of 93%, but poor specificity of 53%. When the cut-off value was increased to 180 U/L, similar to the currently used “gray zone” approach to interpreting Spec cPL® results, the specificity slightly improved reaching 66%, whereas sensitivity decreased to 73%. The authors of that study discussed the possibility of cross-reactivity of non-pancreatic lipases with the substrate. Indeed, a recent study by our group indicates that DGGR is hydrolyzed by other enzymes (likely non-pancreatic lipases; unpublished data). Serum lipase activity in leftover serum samples of 48 dogs with exocrine pancreatic insufficiency (TLI ≤ 1 mg/L) and 66 healthy control dogs was measured using DGGR-based assays (Diazyme Laboratories, Poway, CA and Stanbio Laboratory Boerne, TX). Our data showed that serum lipase activity was within the reference interval in 33 of 48 dogs with EPI using a DGGR-based assay (Figure 1).
| Figure 1:
Serum lipase activity measured by a DGGR-based assay was readily detectable in many dogs with EPI. Conclusion – DGGR-based assays for the measurement of serum lipase activity are not specific for pancreatic lipase. |
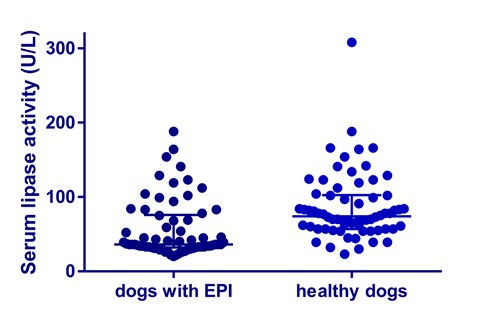 |
| Figure 1. Serum lipase activities in dogs with EPI and healthy control dogs as measured by an assay utilizing DGGR as a substrate.
The center lines show the medians for each group and the whiskers display the interquartile ranges |
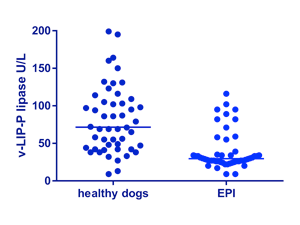
Similarly, we compared serum lipase activity measurements in leftover serum samples from 50 dogs with EPI (TLI ≤ 2.5 mg/L) and 50 healthy control dogs using the v-LIP-P slide (FujiFilm Corporation, Tokyo, Japan) and the Spec cPL® (IDEXX Laboratories, Inc., Westbrook, Maine, USA). While the serum Spec cPL® was in the lower 20% of the reference interval in 49 of 50 (98%) dogs with EPI, serum lipase activity measured with the v-LIP-P slide was in the lower 20% of the reference interval in only 29 of 58 EPI dogs (58%), indicating the detection of lipases other than that of pancreatic origin (Figures 2 and 3). These results clearly show that, in contrast to Spec cPL®, triolein and DGGR are not specific for the measurement of pancreatic lipase.
| Figures 2 and 3:
Spec PL immunoassay concentrations are very low or undetectable in serum from dogs with EPI, but lipase activities are readily detectable and often normal in the same samples. Conclusion – Spec PL is pancreas-specific in origin, but lipase activity as measured by the v-LIP-P slide is not. |
|
 |
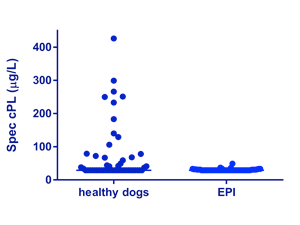 |
| Figure 2: Lipase activity as measured by the v-LIP-P slide in 50 healthy dogs and 50 dogs with EPI. Many of the dogs with EPI had significant serum lipase activities. The lines indicate the medians for both groups. | Figure 3: Pancreatic lipase immunoreactivity as measured by Spec cPL in 50 healthy dogs and 50 dogs with EPI. There is much better separation between healthy dogs and dogs with EPI than for the v-LIP-P slide. The lines indicate the medians for both groups. |
Various studies have been reported that directly compared the aforementioned assays with the Spec cPL® or fPL®.11,14,15 All of these studies found good to moderate correlation between the assays. However, while it is intuitive to assume that serum lipase activity increases as the fraction of pancreatic lipase increases in patients with pancreatitis and that all aforementioned assays will detect that increase in activation, a correlation between tests is not equivalent to a test having the same sensitivity and specificity and thus discriminatory power between disease and controls. A recent study found a moderate agreement (Cohen´s k value 0.803) between a DGGR lipase assay and Spec cPL® when a 2-fold DGGR lipase “gray zone” was applied with cut-offs of 216 U/L for DGGR and 400 mg/L for Spec cPL®. Sensitivity and specificity could not be calculated since pancreatic histopathology was not available.14 Another study compared serum lipase activity as measured with the v-LIP-P slide (FUJI DRI-CHEM 7000V, FUJIF- ILM corporation, Tokyo, Japan) with the measurement of pancreatic lipase using the Spec cPL® and showed good correlation (r=0.91). However, when looking at the data, the graph clearly showed good correlation for lower lipase values, but increasing variation with increasing values, starting at about 400 mg/L Spec cPL® (cutoff for pancreatitis) (see Ishioka, K., Hayakawa, N., Nakamura, K. & Terashima, K. Patient-side assay of lipase activity correlating with pancreatic lipase immunoreactivity in the dog. J. Vet. Med. Sci. 73, 1481–1483 (2011) https://www.jstage.jst.go.jp/article/jvms/73/11/73_11-0166/_pdf). This indicates non-constant variances (heteroscedasticity) and thus a simple correlation does not reflect the assays’ true diagnostic value.
A recent study comparing the DGGR lipase assay (cutoff > 26 U/L) with Spec fPL® (> 3.4 mg/L) in cats with acute or chronic pancreatitis also showed poor agreement between the two assays (Cohen´s k value 0.601)15. Based on histopathology, sensitivity and specificity were 48% and 63% for the DGGR lipase assay and 57% and 63% for Spec fPL®, respectively. However, a previous study showed higher sensitivities and specificities for Spec fPL® in cats with different stages of pancreatitis.16 In cats, with histopathologically confirmed mild pancreatitis, the sensitivity of Spec fPL® was 54%, while this percentage increased to 100% in moderate to severe cases of pancreatitis. The specificity of Spec fPL® has been as high as 100% when tested against a healthy control group. More importantly, the specificity of Spec fPL® reached 67% in cats with clinical signs mimicking pancreatitis but with normal pancreatic histopathology.
Effect of Influence Factors on Results of Lipase Assays
- The triolein-based lipase assay v-LIP-P slide is highly influenced by hemolysis, lipemia, and icterus.
- In contrast, hemolysis, lipemia, and icterus have no effect on the results of the Spec cPL® assay.
In addition to significant problems with sensitivity and specificity, assays for total serum lipase activity have been shown to be greatly influenced by hemolysis, lipemia, and icterus, influence factors that are frequently observed in dogs with pancreatitis and/or other gastrointestinal or hepatic diseases. In an in-vitro study, we recently evaluated the effects of these factors on the performance of lipase activity assays using the substrate triolein, and on the performance of the Spec cPL® ELISA assay. Serum samples that were spiked with canine hemoglobin, Intralipid®, and synthetic ditaurobilirubin, mimicking hemolysis, lipemia, and icterus, respectively (unpublished data). Evaluation of the v-LIP-P slide showed that this assay significantly underestimates serum lipase activity in hemolyzed and icteric samples, thus limiting the sensitivity of the assay in these samples. This is likely to translate into a high false-negative rate in patients with pancreatitis, i.e., missing a diagnosis of pancreatitis in many patients. In lipemic samples the use of triolein dramatically increased the reported lipase activity, potentially leading to a dramatically increased rate of false-positive diagnosis of pancreatitis (poor specificity). In contrast, hemolysis, lipemia, and icterus at the same levels showed no effect on the performance of the Spec cPL® assay. These results show that the Spec cPL® and fPL® are superior for the diagnosis of pancreatitis in dogs and cats to any lipase activity assay, regardless of the substrate that is being used.
References
- Steiner JM, Newman S, Xenoulis P, Woosley K, Suchodolski J, Williams D, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther. 2008;9(4):263–73.
- Trivedi S, Marks SL, Kass PH, Luff JA, Keller SM, Johnson EG, et al. Sensitivity and specificity of canine pancreas-specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med. 2011 Jan 1;25(6):1241–7.
- Strombeck DR, Farver T, Kaneko JJ. Serum amylase and lipase activities in the diagnosis of pancreatitis in dogs. Am J Vet Res. 1981 Nov;42(11):1966–70.
- Polzin DJ, Osborne CA, Stevens JB, Hayden DW. Serum amylase and lipase activities in dogs with chronic primary renal failure. Am J Vet Res. 1983 Mar;44(3):404–10.
- Archer FJ, Kerr ME, Houston DM. Evaluation of three pancreas-specific protein assays, TLI(trypsin-like immunoreactivity), PASP (pancreas-specific protein) and CA 19-9 (glycoprotein) for use in the diagnosis of canine pancreatitis. Zentralbl Veterinarmed A. 1997 Apr;44(2):109–13.
- Quigley KA, Jackson ML, Haines DM. Hyperlipasemia in 6 dogs with pancreatic or hepatic neoplasia: evidence for tumor lipase production. Vet Clin Pathol. 2001;30(3):114–20.
- Parent J. Effects of dexamethasone on pancreatic tissue and on serum amylase and lipase activities in dogs. J Am Vet Med Assoc. 1982 Apr;180(7):743–6.
- Steiner JM, Berridge BR, Wojcieszyn J, Williams DA. Cellular immunolocalization of gastric and pancreatic lipase in various tissues obtained from dogs. Am J Vet Res. 2002 May;63(5):722–7.
- Steiner JM, Rutz GM, Williams DA. Serum lipase activities and pancreatic lipase immunoreactivity concentrations in dogs with exocrine pancreatic insufficiency. Am J Vet Res. 2006 Jan;67(1):84–7.
- Neilson-Carley SC, Robertson JE, Newman SJ, Kutchmarick D, Relford R, Woosley K, et al. Specificity of a canine pancreas-specific lipase assay for diagnosing pancreatitis in dogs without clinical or histologic evidence of the disease. Am J Vet Res. 2011 Mar;72(3):302–7.
- Ishioka K, Hayakawa N, Nakamura K, Terashima K. Patient-side assay of lipase activity correlating with pancreatic lipase immunoreactivity in the dog. J Vet Med Sci. 2011 Nov;73(11):1481–3.
- Panteghini M, Bonora R, Pagani F. Measurement of pancreatic lipase activity in serum by a kinetic colorimetric assay using a new chromogenic substrate. Ann Clin Biochem. 2001 Jul;38(Pt 4):365–70.
- Graca R, Messick J, McCullough S. Validation and diagnostic efficacy of a lipase assay using the substrate 1, 2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′ methyl resorufin)‐ester for the diagnosis of acute pancreatitis in dogs. Vet Clin Path. 2005 Jan; 34(1): 39-43
- Kook PH, Kohler N, Hartnack S, Riond B, Reusch CE. Agreement of Serum Spec cPL with the 1,2-o-Dilauryl-Rac-Glycero Glutaric Acid-(6′-methylresorufin) Ester (DGGR) Lipase Assay and with Pancreatic Ultrasonography in Dogs with Suspected Pancreatitis. J Vet Intern Med. 2014 Mar 5;28(3):863–70.
- Oppliger S, Hartnack S, Riond B, Reusch CE, Kook PH. Agreement of the Serum Spec fPL™ and 1,2-O-Dilauryl-Rac-Glycero-3-Glutaric Acid-(6′-Methylresorufin) Ester Lipase Assay for the Determination of Serum Lipase in Cats with Suspicion of Pancreatitis. J Vet Intern Med. 2013 Jul 26;27(5):1077–82.
- Forman MA, Marks SL, De Cock HEV, Hergesell EJ, Wisner ER, Baker TW, et al. Evaluation of serum feline pancreatic lipase immunoreactivity and helical computed tomography versus conventional testing for the diagnosis of feline pancreatitis. J Vet Intern Med. 2004 Nov;18(6):807–15.
