Texas A&M Researchers Developing New Approach For Analyzing Chemical Mixtures
Drs. Weihsueh Chiu and Ivan Rusyn, professors at the Texas A&M School of Veterinary Medicine & Biomedical Sciences (VMBS), have received one of 11 new research grants from the U.S. Environmental Protection Agency (EPA) as part of an initiative to find novel ways to assess the toxicity of chemical mixtures. While toxicology studies have traditionally […]
Texas A&M University Research Aims To Improve Lyme Disease Diagnostics
Lyme disease, the fastest growing vector-borne illness in the U.S., according to the Bay Area Lyme Foundation, is challenging to diagnose and can only be treated in the early stages of infection. Once the infection spreads to the nervous and muscular systems, it is both harder to detect and less susceptible to antibiotics. Research by […]
Aggie Pathologists Represent Texas A&M, Win Award At National Meeting
More than a dozen representatives of the Texas A&M School of Veterinary Medicine & Biomedical Sciences (VBMS) recently attended and brought home an award from the 2022 annual meeting of the American College of Veterinary Pathologists (ACVP) in Boston. From Nov. 12-15, a group of VMBS faculty, residents, and students attended the internationally recognized, One […]
Legere Receives Award For Excellence In Graduate Research
Dr. Rebecca Legere, a doctoral student in the Department of Large Animal Clinical Sciences at the Texas A&M School of Veterinary Medicine & Biomedical Sciences (VMBS), was recently honored as a 2022-2023 recipient of the Ethel Ashworth-Tsutsui Memorial Award for Research. The award recognizes graduate students who distinguish themselves in research and is offered in […]
Texas A&M Superfund Center Receives NIH Funding Renewal To Continue Research on Climate Change, Health
The Texas A&M University Superfund Research Center, housed within the School of Veterinary Medicine & Biomedical Sciences (VMBS), has received a five-year renewal of its funding from the National Institute of Environmental Health Sciences (NIEHS), one of the National Institutes of Health (NIH). Since its launch in 2017, the Superfund Center has focused on understanding […]
VMBS Researcher Studying Ticks To Ensure Safer Future For People, Animals
Dr. Albert Mulenga, a professor in the Texas A&M School of Veterinary Medicine & Biomedical Sciences’ (VMBS) Department of Veterinary Pathobiology (VTPB), has found the research that makes him tick—ticks and tick-borne diseases. Because these parasites pose a global threat to human, animal, and environmental health, Mulenga has taken a One Health approach to his […]
Texas A&M Study Suggests Father’s Alcohol Consumption Has Consequences During Conception
Researchers found that the mother’s placenta is healthier if the father has limited or no alcohol consumption during conception. A father’s alcohol consumption may affect the mother’s placenta and, in turn, the unborn child, according to a new study from Texas A&M University’s School of Veterinary Medicine and Biomedical Sciences (VMBS). Published Aug. 11 in […]
Texas A&M Biomedical Sciences Students Launch Research Program In McAllen
Since its inception, one of the key selling points at the Texas A&M Higher Education Center in McAllen has been that students will have the same opportunities they have in College Station. This also has been true for the School of Veterinary Medicine & Biomedical Sciences’ (VMBS) Biomedical Sciences (BIMS) program at the Higher Education […]
Texas A&M Veterinary Students Complete Annual Research Training Program
Fifteen veterinary students from the Texas A&M School of Veterinary Medicine & Biomedical Sciences (VMBS) spent the summer learning about research and scientific communication as participants in the 2022 Veterinary Medical Scientist Research Training Program (VMSRTP). VMSRTP is a 13-week program during which students conduct full-time research under the advice and direction of a faculty […]
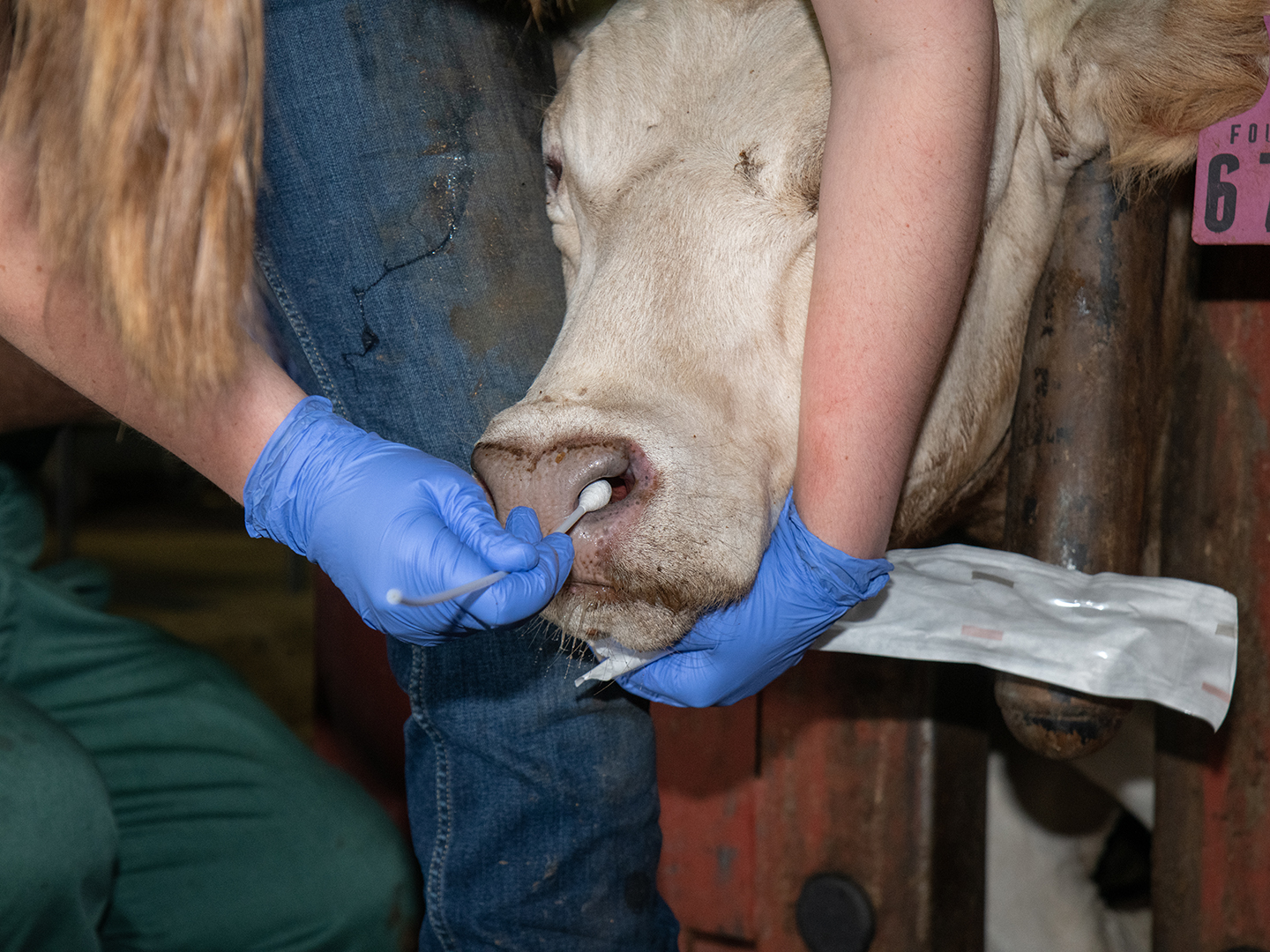
VERO Researcher Studies Common Pathogen To Improve Diagnosis, Treatment Of Bovine Respiratory Disease
One of the primary research areas for the Texas A&M School of Veterinary Medicine & Biomedical Sciences’ (VMBS) Veterinary Research, Education, and Outreach (VERO) program is trying to solve the complex problem of Bovine Respiratory Disease (BRD). Though research has been ongoing for decades, BRD still accounts for more than half of all feedlot deaths […]