NR4A receptors
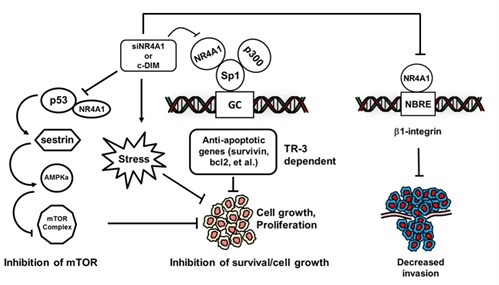
The nuclear receptor NR4A1 (Nur77/TR3) is an orphan receptor with no known endogenous ligands; however, this receptor plays an integral role in cellular homeostasis and disease. NR4A1 is overexpressed in multiple solid tumors and is a negative prognostic factor for cancer patients; moreover, functional studies demonstrate that NR4A1 exhibits pro-oncogenic activity and plays an important role in cancer cell growth and survival. Previous studies in pancreatic, lung, colon, kidney and breast cancer cell lines have identified three major pro-oncogenic NR4A1-regulated pathways that are inhibited by 1,1-bis(3’-indolyl)-1-(p-substituted phenyl) methane (C-DIM) compounds which are NR4A1 ligands that act as antagonists. Current studies are focused on the antineoplastic activities of NR4A1 antagonists in multiple solid tumors and identification of key oncogene-like factors regulated by NR4A1 & targeted by our novel NR4A1 antagonists. In addition, we are currently screening C-DIMs against NR4A2 & NR4A3 and determining their effects on NR4A-dependent inflammation, neurological and metabolic functions.
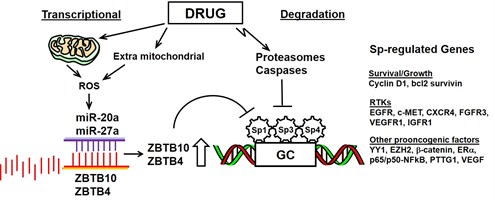
Specificity protein (Sp) transcription factor
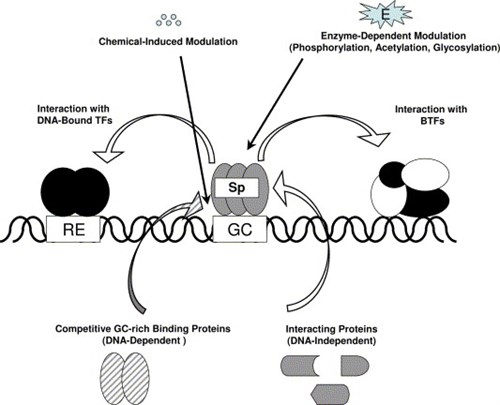
Specificity protein (Sp) transcription factors (TFs) are members of the Sp/Kruppel-like factor family, and Sp proteins play an important role in embryonic and early postnatal development. Sp1 has been the most extensively investigated member of this family, and expression of this protein decreases with age, whereas Sp1 and other family members (Sp3 and Sp4) are highly expressed in tumors and cancer cell lines. AREA COVERED: The prognostic significance of Sp1 in cancer patients and the functional pro-oncogenic activities of Sp1, Sp3 and Sp4 in cancer cell lines are summarized. Several different approaches have been used to target downregulation of Sp TFs and Sp-regulated genes, and this includes identification of different structural classes of antineoplastic agents including NSAIDs, natural products and their synthetic analogs and several well-characterized drugs including arsenic trioxide, aspirin and metformin. The multiple pathways involved in drug-induced Sp downregulation are also discussed. EXPERT OPINION: The recognition by the scientific and clinical community that experimental and clinically used antineoplastic agents downregulate Sp1, Sp3 and Sp4, and pro-oncogenic Sp-regulated genes will facilitate future clinical applications for individual drug and drug combination therapies that take advantage of their unusual effects.
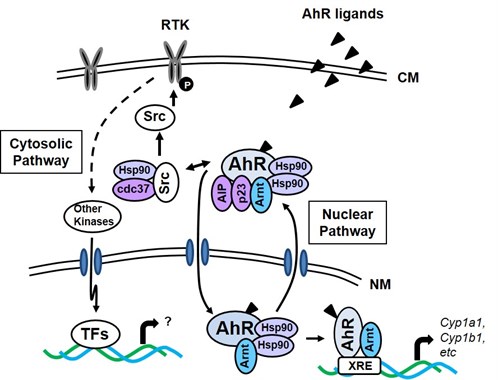
The Ah receptor
The aryl hydrocarbon receptor (AHR) binds with high affinity to the environmental toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and with moderate to low affinity to structurally diverse health-promoting phytochemicals, endogenous biochemicals, and several pharmaceuticals that are clinically used for treating multiple disorders. The molecular mechanism of action of AHR ligands has been extensively investigated, and ligand-dependent activation of the AHR generally results in formation of a nuclear AHR-AHR nuclear translocator heterodimer, which binds dioxin responsive elements (DREs) in target gene promoters to activate transcription. We are currently investigating the mechanisms associated with selective Ah receptor modulator- induced inhibition of cancer cell migration and invasion and also the selective Ah receptor agonist/antagonist activity of Ah receptor active microbiota-derived compounds.
Long non-coding (lncRNAs)
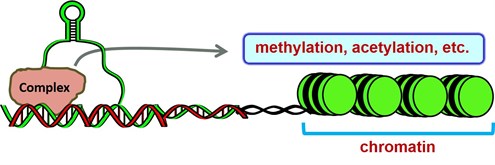
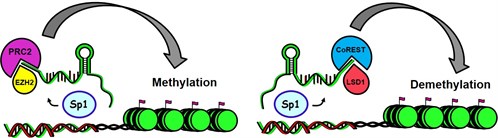
Long non-coding RNAs (lncRNAs) contain >200 nucleotides and the ENCODE Project Consortium have identified 927 lncRNA genes that produce 14,880 transcripts. The biological roles of only a small fraction of lncRNAs have been reported but it is apparent that they exhibit multiple functions and contribute to cellular homeostasis and diseases including cancer. LncRNAs can interact with RNA, DNA and proteins, and their mechanisms of action include their activity as decoys and guides that titrate away or guide proteins to their presumptive DNA targets, respectively. LncRNAs can also bridge nuclear protein-DNA interactions from distal sites and act as scaffolds that facilitate protein/RNA/DNA interactions at specific sites including chromatic. Studies in this laboratory have primarily focused on lncRNAs in pancreatic, liver cancer and rhabdomyosarcoma cells (ongoing) and our results have characterized their oncogenic activity and potential as drug targets. Ongoing studies are addressing hypothesis that metastasis-associated-in-lung-adenocarcinoma-transcript-1 (MALAT-1) is pro-oncogenic in pancreatic cancer cell and in animals models and that MALAT-1 expression is regulated by specificity protein (Sp) transcription factors and can be targeted by anticancer agents that downregulated Sp proteins.