Frequency Variation… 2023 Antimicrobial Agents and Chemotherapy article
Title: Frequency Variation and Dose Modification of Benznidazole Administration for the Treatment of Trypanosoma cruzi Infection in Mice, Dogs, and Nonhuman Primates
Authors: Juan M. Bustamante, Brooke E. White, Gregory K. Wilkerson, Carolyn L. Hodo, Lisa D. Auckland, Wei Wang, Stephanie McCain, Sarah A. Hamer, Ashley B. Saunders, and Rick L. Tarleton
Journal/Date of Publication: Antimicrobial Agents and Chemotherapy, 2023
DOI: 10.1128/aac.00132-23
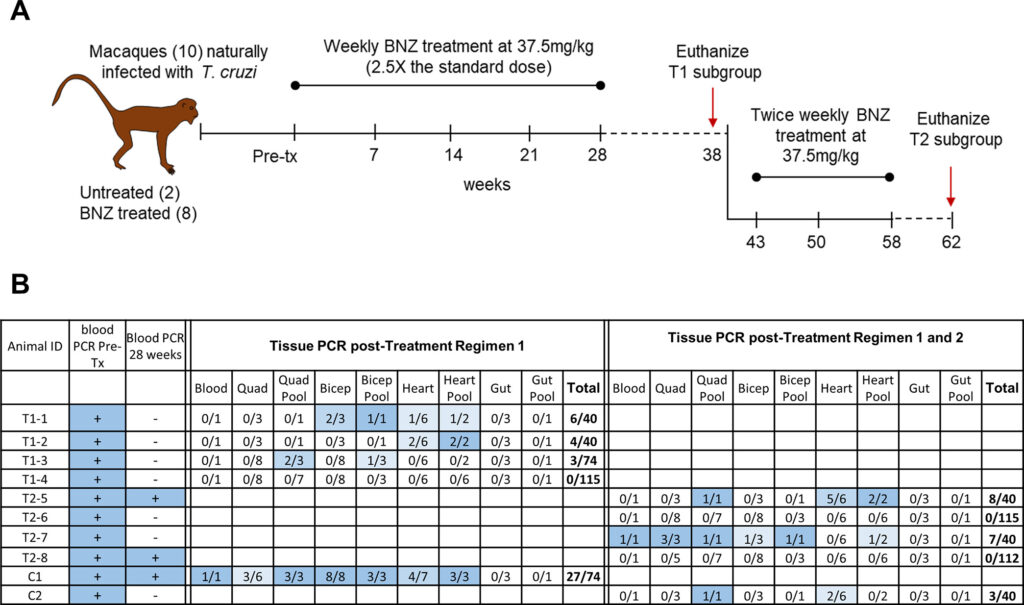
Objective: Treatment of chronic Trypanosoma cruzi (Chagas disease) infections in animals, with a focus on the effectiveness of higher dose, intermittent benznidazole treatment protocols.
Type of Study: Prospective
Conclusions:
- Twice-weekly high-dose benznidazole treatment over several months led to parasitological cures in some animals (mice, dogs, and macaques), though results varied.
- Shorter or less frequent treatment regimens were less effective.
- Parasites did not develop resistance to benznidazole even after failed treatment regimens, and re-treatment was still effective.
- The protocol has potential applications for high-risk animals (example: working dogs, zoo animals), but its utility in humans remains uncertain due to the risk of adverse effects.
Clinical application:
- Higher benznidazole doses, administered intermittently, can cure T. cruzi in mice, dogs, and macaques.
- The twice-weekly regimen is promising for chronically infected dogs and non-human primates, providing an alternative to daily dosing protocols.
- Retreatment is viable if initial protocols fail, as parasites did not exhibit resistance.
- Dogs showed declining antibody levels, suggesting effective parasite clearance.
- The protocol could be especially beneficial for high-value animals in endemic regions.
- Long-term, high-dose benznidazole treatment shows limited toxicity in dogs and non-human primates, making it a potential option in veterinary settings.
- The absence of uniform outcomes suggests treatment duration may need to be tailored to individual cases.