A Fertile Field
Could discoveries made by CVM professor and Patsy Link Chair Katrin Hinrichs lead to healthier in vitro-produced human embryos?
Story by Callie Rainosek & Dr. Megan Palsa
Horses have played a vital role in world history.
Until about a century ago, they were one of mankind’s main sources of transportation and communication.
However, thanks to advancing technology, many horses have transitioned into companion animals or are specially trained to compete in events or perform specific tasks.
Mankind’s relationship with horses has certainly changed over the years, and thanks to Dr. Katrin Hinrichs at the Texas A&M College of Veterinary Medicine & Biomedical Sciences (CVM), our relationship with horses could change even more.

HORSING AROUND WITH ICSI
A member of the CVM’s Department of Veterinary Physiology & Pharmacology, with a joint appointment in the Department of Large Animal Clinical Sciences, Hinrichs is a professor and the Patsy Link Chair of Mare Reproductive Studies. She is recognized internationally for her research in equine reproductive physiology and oversees one of the few labs in the world capable of performing intracytoplasmic sperm injection (ICSI) in the horse, a process that is now the standard in equine assisted reproduction.
ICSI is a more complex form of traditional in vitro fertilization and is the only process that can efficiently produce a fertilized equine embryo outside of a mare’s body. The process involves picking up a single sperm in a pipette, under a powerful microscope, and inserting the sperm into a mature oocyte—or unfertilized egg.
Given the right conditions, this will produce a fertilized egg and an early embryo will develop.
There are two ways to perform ICSI—the conventional method and a specialized method that involves piezoelectricity. In conventional ICSI, a pointed glass pipette is used to “roll” the sperm against the bottom of a manipulation dish to rupture its outer membrane, which aids in fertilization. The sperm is then injected through the zona, or the outer “shell” of the oocyte, and then through the oocyte’s membrane for fertilization.
In “piezo” ICSI, the pipette is blunt, and the shaft of the pipette is encased in a small motor unit that transmits minute vibrations to the pipette, which allow the pipette to act like a drill. With piezo ICSI, the motions of the drill are used to rupture the sperm membrane, and the pipette then drills a miniscule hole in the zona. The pipette is placed through the hole and the sperm is injected through the oocyte’s membrane into the oocyte.
Many labs that perform ICSI—in both humans and horses—use the conventional method since piezo ICSI requires additional expertise and the equipment is more expensive. Additionally, piezo ICSI has traditionally used mercury in the pipette to act as a stabilizer and limit pipette movement. Since mercury has the potential to be toxic to embryos, many labs prefer conventional ICSI.
However, when Hinrichs’ lab at Texas A&M started working on equine ICSI in 2001, they opted to use the piezo drill because of its greater success in fertilizing embryos. Hinrichs’ lab was the first to report efficient in vitro development of equine embryos after ICSI and remains one of the top laboratories in the world for equine embryo production.
Since no critical research had ever been done to compare embryo production rates between conventional and piezo ICSI, Hinrichs always questioned whether the success of her lab using piezo was due to better lab conditions overall, or because piezo ICSI was more efficient in producing embryos than is conventional ICSI.
Hinrichs got the chance to answer this question about two years ago when a scientist named Renato Salgado started working in her lab.
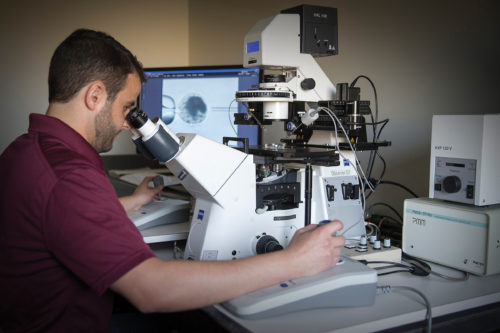
A ‘GARDEN’ OF EMBRYOS
“We were in this optimum situation,” Hinrichs said, “because Dr. Salgado had experience performing conventional ICSI on humans.”
While standard in vitro fertilization methods (mixing of sperm and oocytes together in a Petri dish) work in humans, embryologists have found that fertilization rates are higher when sperm is injected into the oocyte via ICSI. Therefore, many assisted-reproduction clinics for humans now use ICSI, and this is typically done using the conventional method.
Salgado’s expertise in conventional ICSI was combined with the skills of Dr. Joao Brom-de-Luna, another scientist in Hinrichs’ lab who specializes in piezo ICSI.
Hinrichs designed a study in which the two scientists fertilized equine oocytes side-by-side in the lab, using the two ICSI methods. The development of the resulting embryos was then compared. To make the results applicable to human laboratories, Brom-de-Luna worked with a non-toxic compound instead of mercury in the piezo pipette.
“We collected oocytes and matured them in the same incubator,” Hinrichs explained. “Then we divided them into two groups—one group went to Renato for conventional ICSI, and one went to Joao for piezo ICSI. We cultured the oocytes in the same incubators afterwards and were surprised by the results.”
Hinrichs and her team were expecting to find that one method produced more transferrable embryos than the other, meaning that the embryos were at a stage in development where they can be transferred to a recipient mare to form a potential pregnancy.
Instead, Hinrichs and her team found that the piezo and conventional ICSI methods produced the same number of transferrable embryos. However, the embryos produced using the conventional method developed more slowly.
“It’s like if you planted seeds in a garden,” Hinrichs explained. “The seeds may come up between seven and 10 days after you plant them, but some seeds will come up at day seven, some at day eight, and some at day nine or even 10. The embryos that develop earlier are healthier—they are more likely to make a pregnancy after you transfer them to a mare. The embryos produced using piezo ICSI grew faster and most developed to a transferrable stage by day seven. The embryos produced using conventional ICSI took eight or nine days.”
This discovery led to even more questions.
Hinrichs and her team hypothesized that the action of the piezo drill on the sperm membrane may have something to do with why the piezo ICSI method led to faster embryo development, possibly because the drill was rupturing the sperm’s acrosome.
“The acrosome is essentially a bag of enzymes that the sperm carries at the tip of its head,” Hinrichs said. “During natural fertilization, these enzymes are released to help the sperm get through the zona, the outer ‘shell’ of the oocyte. The real oocyte is inside this shell, so in natural fertilization the sperm no longer has the acrosome when it actually contacts the oocyte. However, when we do ICSI, we just pick up the sperm, acrosome and all, and inject it.”
Hinrichs thought that the piezo ICSI method may have been rupturing the sperm acrosome before injection of the sperm into the oocyte. If this was true, then piezo ICSI mimicked the natural fertilization process more closely than did conventional ICSI, potentially allowing the piezo embryos to develop faster.
To test her hypothesis, Hinrichs and her team arranged for another examination of embryo development. The team performed more rounds of side-by-side ICSI, but used special dyes to stain the injected oocytes to observe the sperm within the oocyte closely in the 18 hours after ICSI.
“Immediately after ICSI, we were anticipating that we would see that the acrosome was gone in the piezo treatment and was present in the conventional treatment,” Hinrichs said. “However, that wasn’t true. Both piezo and conventional ICSI sperm still had their acrosomes when we looked at them immediately after injection into the oocyte. But at six hours after ICSI, we saw this huge difference in the piezo treatment. The acrosome had come off, the sperm’s head was starting to swell, and fertilization was about to take place. However, after six hours in the conventional ICSI treatment, the acrosome was still on the sperm, and the sperm head showed no sign of swelling.”

STUMBLING ON A DISCOVERY
“If the development process of both types of embryos is so different, why is there no difference in embryo production rates?” Hinrichs questioned.
Hinrichs investigated further and stumbled on a discovery.
“We began to compare the quality of the transferrable embryos,” Hinrichs explained, “and the conventional embryos were significantly lower quality compared to the piezo embryos.”
Hinrichs determined embryo quality based on the number of nuclei in the embryo and the percentage of nuclei that were developing normally. Abnormal nuclei appeared fragmented, indicating that some cells within the embryo were dying.
“The conventional embryos had significantly lower nucleus numbers and higher rates of nuclear fragmentation,” Hinrichs explained. “In other words, there were fewer cells in the conventional embryos overall and more of those cells were abnormal compared to piezo embryos.”
This difference in quality is important because it could determine if the embryo leads to a successful pregnancy.
A NEW MODEL FOR ASSISTED REPRODUCTION IN HUMANS
Horses are one of the few species in the world in which, like humans, conventional ICSI is repeatedly successful in producing embryos in the lab.
Therefore, Hinrichs believes that horses are the closest models for humans when it comes to assisted reproduction, and her findings, then, are significant for both equine scientists and for laboratories working in human assisted reproduction techniques.
“All of this is interesting because conventional ICSI is used in humans,” Hinrichs said. “However, we showed that in the horse, while conventional ICSI also works to produce embryos, the embryos produced using piezo ICSI develop more normally and are better quality.”
Hinrichs suggests that piezo ICSI could be a better way to produce human embryos for human assisted reproduction. However, little work has been done on piezo ICSI in humans because it usually uses mercury in the pipette.
“Of course, you’re not going to use mercury with a human embryo; it is potentially toxic,” Hinrichs explained. “But in our study, we used a non-toxic substitute for mercury, a carbon-based compound called fluorinert. Fluorinert is also safe to be used in humans.”
Despite the success of her study using a compound that is safe to use on human embryos, Hinrichs recognizes that further research is needed before piezo ICSI can be regularly performed on human embryos. Additionally, Hinrichs hopes that future research will explore if the healthier embryos produced through piezo ICSI lead to more successful pregnancies.
“Something as simple as using a different ICSI technique could help produce higher-quality embryos, which, in turn, could lead to more successful human pregnancies,” Hinrichs said.
Hinrichs’ research was published in the Journal of Assisted Reproduction and Genetics—a scientific journal that focuses on assisted-reproduction technologies in humans and associated research in relevant animal models.
Publishing her research in this human-based journal further solidified the novelty of her study and the significance of the horse being used as a model for assisted reproduction in humans.
SAVING ENDANGERED SPECIES

Hinrichs may have a strong passion for equine reproductive physiology, but she has goals to help other species reproduce, too. Over the years she has looked for ways to use her expertise to help endangered species, such as the northern and southern white rhino and the Grevy’s zebra.
“My next dream is to use assisted reproduction to help widen the gene pool of endangered species in the United States,” Hinrichs said.
But before pursuing this goal, she is doing more research to study basic factors that might play a role in the health of equine embryos, such as incubator temperature, atmosphere, and pH level. Although there are now methods for performing equine ICSI that many laboratories follow, nobody in the field of equine assisted reproduction has critically tested the basic requirements for optimum development of the equine embryo.
“We want to make sure that we’re producing embryos at the best rate possible and that they are as high quality as we can get,” Hinrichs said. “All of these little factors may affect the health of our embryos and, therefore, affect successful pregnancy rates.”
A PIONEER IN THE FIELD
Hinrichs was just a little girl when she first discovered her love for horses. Now, she is one of the leading pioneers in equine assisted reproduction.
For more than 30 successful years, she has not only improved assisted reproduction in horses, she has now brought humans and horses closer together than ever before.
###
Note: This story originally appeared in the 2019 Spring edition of CVM Today.
For more information about the Texas A&M College of Veterinary Medicine & Biomedical Sciences, please visit our website at vetmed.tamu.edu or join us on Facebook, Instagram, and Twitter.
Contact Information: Jennifer Gauntt, Interim Director of Communications, Media & Public Relations, Texas A&M College of Veterinary Medicine & Biomedical Science; jgauntt@cvm.tamu.edu; 979-862-4216


