Proventricular Dilatation Disease PDD, is a significant cause of disease and death in captive parrots and some wild birds.
Our major task is to learn more about this virus and to seek new methods to prevent, diagnose, and treat the diseases caused by this virus.

PDD was first described in the late 1970s in young macaws imported from the Santa Cruz area of Bolivia and that is why was originally called “Macaw Wasting Disease”. It is suggested that it was a new disease in macaws and other large psittacines species because of its high lethality. Today, it has been found in over 50 different species of psittacines and a number of other birds from canaries to waterfowl. Unfortunately, there is no effective cure for PDD research continues to look for more effective ways to mitigate, slow, or prevent the development of GI or neurological symptoms of PDD/AGN.
The name PDD comes from a common clinical outcome: dilation of the proventriculus (widening of the region of the stomach between the crop and the gizzard). This dilation is caused by accumulated food due to partial paralysis of the digestive system. ABV infections can affect multiple body systems including the central nervous system and the GI tract. Other conditions can mimic some or all of the symptoms of PDD. While some ABV infected birds develop PDD, many birds who test positive for ABV do not develop any symptoms. It is currently unknown what triggers disease progression after infection and continues to be an area of active research investigation.
Prevention:
The Psittacine Bornaviruses are a family of viruses found worldwide. Since their discovery, it has become clear that they are common in captive parrots but do not necessarily cause disease.
The virus is not highly resistant to environmental degradation. Schubot Center research under the leadership of Dr. Jeffrey Musser have shown that it can be readily destroyed by drying. It also appears to be susceptible to destruction by commonly employed disinfectants.
Vaccination against this virus is being studied at the Schubot Center at the present time. It is too soon to say whether this will be successful but preliminary studies with some innovative new vaccines have yielded encouraging results.
Back in 2010, these studies were initially undertaken by Dr. Susan Payne and her graduate student Samer Hameed as well as Dr. Musser’s graduate student Tariq Hantash.
Since June 2021, Dr. Caitlin Mencio has joined our research team as an Assistant Research Scientist thanks to the generosity of the Pat Palmer Foundation and the support of the Schubot Endowment. Dr. Mencio is currently leading the Schubot Avian Borna Virus 9ABC) research team. Her research will be focused on virus transmission, disease diagnosis, and vaccine development. She is particularly interested in the molecular basis for ABV infection and the progression from infection to clinical symptoms of PDD
Diagnosis:
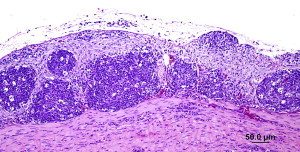
There are two ways to diagnose viral infections. One is to look for the virus itself, the other is to determine if a bird has made antibodies to the virus. We have been able to detect Bornaviruses in bird droppings. Dr. Heatley’s studies have shown that the virus infects the bird’s kidneys and hence is spread in the urine (the white portion of a bird dropping). Not all birds, however, develop kidney infections and hence do not always shed the virus in their urine. Thus this is not an ideal test. We have been investigating antibody-based tests for detecting infected birds and are obtaining encouraging results with modern rapid testing technology. Watch this space for new results as we get them.
Treatment:
Bornaviruses are the cause of much suffering in birds and it is essential that we seek ways of treating sick birds. Both Dr. Hoppes and Dr. Musser are investigating drugs that might destroy the avian bornavirus or somehow stop the progress of the disease. Results to date are mixed but we will continue to support this approach.
Epidemiology:
While disease in captive birds is our main focus, we have also investigated the presence of these viruses in wild birds. Thus Waterbird bornaviruses occur in about 25% of mute swans, 15% of geese and 10% of wild ducks in this country (it’s also present in domestic ducks). Current evidence suggests that these viruses do not transfer between waterbirds and parrots. There is no evidence that the avian bornaviruses can infect mammals.
Schubot Center researchers have been studying this disease and the virus that causes it for many years. This web site is designed to keep you up-to-date with our recent research information.
Vaccination against this virus is being studied at the Schubot Center at the present time. It is too soon to say whether this will be successful but preliminary studies with some innovative new vaccines have yielded encouraging results.
Answers to Frequently Asked Questions about PDD and Parrot Bornavirus
Due to the impacts of Avian Bornavirus on captive parrots and the confusion surrounding PDD, Avian Bornavirus, Avian Gangleoneuritis and related topics, members of the Schubot Center for Avian Health have put together this AVB FAQ sheet to help members of the parrot owner community better understand some of the complexities surrounding these topics.
The information is broken down in to four sections: General Information, Testing, Disease, and Supporting Literature. The topic is very complex and this document is unable to address all these complexities as much is still unknown or debated by experts in the field.
For more information, the reader can refer to the scientific papers cited throughout the PDD and Avian Bornavirus FAQ document and listed in the Supporting Literature section.
General Information
My bird tested positive for avian bornavirus (ABV). Now what?
A positive ABV test is NOT a death sentence. Many birds test positive for ABV and live full, happy lives and never show any signs of disease. Speak with your veterinarian about possible next steps, but if your bird has no clinical symptoms then no action beyond routine checkups may be needed.

What is Avian Bornavirus (ABV)?
Avian Bornavirus (ABV) is a negative, single-strand RNA virus found in birds that preferentially targets the nervous system. It has been found in over 80 avian species worldwide including all major groups of parrots.
What are the outcomes of ABV infection?
ABV infection has markedly variable outcomes in birds.
ABV positive birds may:
- Show no outward symptoms
- Have no neurological or physiological deficits
- Live long, healthy lives
However, ABV infection may result in the following:
- The development of Proventricular Dilation Disease (PDD) also known as Avian Ganglioneuritis (AG) or Avian Bornaviral Ganglioneuritis (ABG).
- Acute behavioral changes
- Neurological disorders
- Movement disorders
- Feather-damaging behavior
- Heart disease
If an ABV-positive bird begins to exhibit any of these symptoms, please consult with your veterinarian as soon as possible.
What are Proventricular Dilation Disease (PDD) and Avian Ganglioneuritis (AG)?
The name PDD stems from an early recognized clinical outcome of infection with avian Bornavirus: dilation of the proventriculus (widening of the region of the stomach between the crop and the gizzard) and proximal small intestine. This dilation is caused by accumulated food due to partial paralysis of the digestive system. However, dilation of the proventriculus can also occur with other diseases, including heavy metal toxicity, bacterial enteritis, foreign bodies and parasitic infections.
Avian Ganglioneuritis is another name for the disease that can result from ABV infection. However, Avian Ganglioneuritis in the absence of infection with ABV has occurred. It is possible that other infections that cause increases in gangliosides may also trigger a similar response and cause the development of PDD/AG. This has led to disagreement about naming the disease as many systems in the body can be affected by ABV, some clinical cases lack proventricular dilation and other diseases can cause ganglioneuritis. All the terms PDD, AG, and avian bornaviral ganglioneuritis (ABG) can be found in the literature when discussing disease resulting from ABV infection.
What is the cause of PDD/AG?
PDD/ABG/AG is caused by an inflammatory response of the nervous system which leads to problems in the gastrointestinal tract, peripheral and central nervous systems, and heart. While ABV infection has been shown to be a factor in the development of PDD/AG, many birds infected with ABV live long, healthy lives with no evidence of disease. Instead, it is possible that ABV infection needs to be paired with some other (currently unknown) factor to cause disease.
What are the clinical symptoms of PDD/ABG?
Common clinical symptoms include:
- Gastrointestinal issues:
- Weight loss
- Crop stasis
- Proventricular and intestinal enlargement
- Regurgitation
- Passage of undigested food in feces
- Central nervous system issues:
- Tremors and seizures
- Motor problems (inability to fly, loss of muscle control)
- Blindness
- Heart disease
- Myocarditis
- Arrythmias
- Heart block
Is there a test for PDD/AG?
Yes. The two most commonly used types of tests for ABV are PCR tests and serologic antibody tests. The PCR tests detect the presence of RNA from Avian Bornavirus in the sample. Tests can be run on fecal matter, tissue samples, or cloacal swabs from birds. The antibody tests use serum from blood to test for antibodies against viral proteins or gangliosides.
What is the difference between the two types of tests?
The PCR test, in the case of fecal samples or swabs, will tell you if the bird is actively shedding virus into the environment. However, viral shedding is not constant in infected birds so a negative test alone does not indicate a lack of infection.
The serologic antibody test tells you if the bird currently has immune factors (antibodies) that target either against ABV proteins circulating in the blood or gangliosides which have been found to be increased in birds exhibiting AG. The presence of ABV antibodies indicates exposure of the bird to the virus in the past while a positive ganglioside test indicates possible ganglioneuritis. However, the serological antibody test alone does not provide information about whether the bird is actively shedding virus.
If my bird tests positive for ABV does that mean they will get sick or die from PDD/AG?
No! Many birds that test positive for ABV live long and happy lives, never developing PDD/AG. If a bird tests positive but is otherwise healthy, keep an eye out for the development of any clinical symptoms and seek veterinary attention as soon as you notice them. Inform your veterinarian that your bird has tested positive for ABV in the past when discussing any new symptoms.
There is some evidence that the ganglioside serologic testing may provide more definitive diagnostic information for PDD/AG. For some veterinarians, this is the preferred method of testing for PDD/AG in parrots.
I have multiple birds, what should I do if one tests positive for ABV?
Remember, just because a bird tests positive for ABV does NOT mean they will ever develop disease or spread it to nearby birds. That being said, there are precautions that can be taken to minimize the possibility of viral transmission. These include:
- Separation of ABV positive birds from ABV negative birds
- Taking precautions to reduce the risk of cross-contamination
- Don’t share cages, toys, or food/water dishes between ABV positive and ABV negative birds,
- Work with ABV negative birds before working with ABV positive birds or shower after working with ABV positive birds
- Thoroughly clean items that come into contact with ABV positive birds (cages, dishes, perches, cage covers, toys, etc.)
However, we recognize this is not always possible. When it is not, owners must weigh the pros and cons of maintaining groups of birds with mixed ABV-status.
Euthanasia is not the only option for birds that test positive for ABV but it can be discussed with your veterinarian, especially in the case of a bird with severe disease that is unresponsive to treatment and supportive care.
Why do some birds that are ABV positive never develop PDD/AG?
Currently we do not know what triggers disease progression from infection with ABV to full PDD/AG. Research into why this happens and how we can prevent it is ongoing.
Is there a treatment for PDD/AG?
Unfortunately, there is currently no effective cure for PDD/AG. Treatment includes supportive care that addresses symptoms and nonsteroidal anti-inflammatory drugs. Research continues to look for more effective ways to reduce or prevent ABV infection and mitigate the development of GI or neurological symptoms of PDD/AG after infection.
Is there a vaccine for ABV?
There is currently no vaccine against ABV. The Schubot Center and other research institutions are currently conducting research into the creation of a safe and effective vaccine.
Testing

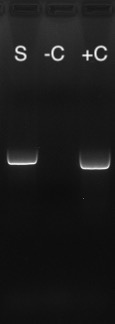
Which test is best, PCR or Serological Antibody?
A PCR test performed on samples of droppings is an effective way to check if your bird is actively producing virus, meaning they could spread it to other birds including their offspring. However, in the case where you want to know if a bird has ever previously been exposed to the virus, the antibody testing may be useful.
While not testing specifically for ABV, gangliosides have been shown as a possible marker for disease progression in birds with PDD/AG. Serological testing for anti-ganglioside antibodies is being examined as a possible method for more accurate detection of clinically affected birds. Consult with your veterinarian to determine if this test would be beneficial for your bird.
Where can I get an ABV test for my bird?
A number of commercial labs perform PCR testing for ABV, including the Schubot Center. The Schubot center offers both PCR and ABV antibody serological testing for a fee.
For more information, contact us.
What samples are needed for the PCR test? How should I collect them?
Each lab will have their own sample collection recommendations. We encourage you to check the website of the lab you are planning to utilize for more information. For our analysis, the Schubot Center for Avian Health recommends:
- Individual Bird:
- Freshly collected droppings, or a cloacal swab, from 3 different days within a 7-21 day period. These samples should be stored in a sealed container in the refrigerator, to prevent them from drying out, until they are sent for testing.
- Multiple birds/Aviary setting:
- Freshly collected droppings from all birds from 3 different days over a 7-21 day period stored in a sealed container in the refrigerator. If the pooled sample comes back positive, individual birds can then be tested, either by droppings or cloacal swabs.
How reliable are the tests for ABV and how do I interpret test results?
PCR tests:
PCR testing for ABV is very unlikely to present a false positive result. If the PCR test is positive, then ABV is likely present in the bird. However, as mentioned above, this does NOT mean your bird will develop disease.
A negative PCR test from a fecal sample or cloacal swab can be a bit harder to interpret. It means your bird was not shedding the virus on the day(s) the samples were collected. This could be because (A) your bird is not infected with the virus or (B) the virus was not being actively shed but is still present in the animal. Because of this intermittent shedding, sending multiple samples for PCR testing may be useful for determining if a bird is infected.
If your birds have a long history of being healthy and minimal exposure to other birds and you receive negative PCR tests, you are likely correct in assuming your birds are not infected with ABV. However, if your bird has symptoms and you suspect your birds may be infected with ABV, consult with your veterinarian regarding additional diagnostic testing.
Antibody tests:
Serologic anti-ABV antibody tests provide very different information from PCR tests. A positive antibody test shows that the bird has antibodies against ABV. This suggests that the bird has been exposed to ABV in the past and has mounted an immune response. However, it does not tell you if the birds are currently infected or not.
A negative antibody test indicates that the bird does not have antibodies against ABV. However, this could mean two very different things. It could be that the bird has never been exposed to or infected with ABV. Alternatively, it could be that the bird has been infected with ABV but that the bird has not yet mounted an immune response to the virus. For these reasons, using multiple testing methods may provide a more conclusive answer about ABV infection in your bird.
Disease



How does ABV cause disease?
Most of the damage caused by PPD/AG is thought to be caused by an autoimmune response. After infection, the virus enters cells in the nervous system and GI tract of birds. In sick birds, lymphocytes (white blood cells) are often found concentrated around the sites where there is damage. This, paired with the lack of cell death of infected cells in culture, suggests that the damage may be caused by the birds’ own immune system attacking and damaging the bird’s own cells. This idea that the infection by the virus is not causing cell damage is also supported by data from studies with mammalian borna disease virus.
Are there different types of bornavirus?
Yes, there are a variety of different types of bornavirus that infect different types of hosts. Currently at least 17 different strains have been found in birds of which seven have been identified in parrots. There are also different strains that have been found in geese, canaries, and waterfowl. To date, no strain of ABV has been reported as being passed from birds to humans. Most of these strains are believed to be found in a relatively narrow range of avian hosts, but more research is needed on this subject.
Borna disease virus (BDV) infects mostly mammals and is found predominately in central Europe. BDV causes brain inflammation (encephalitis) in sheep, horses and other domestic mammals and has been known to infect humans.
How is ABV spread?
Transmission of ABV is not currently well understood as studies in laboratory settings often result in inconsistent results. As such, scientists have proposed several possible methods for transmission. Oral transmission has been proposed to occur when birds inadvertently consume feces from infected birds that contain the virus. However, researchers have been unable to infect birds experimentally with virus given orally, indicating that fecal oral infection may not be that common, and damage to the epithelium (skin) may be needed for infection to occur. The virus has also been found in the lungs of infected birds and has been detected in the air of an infected aviary, indicating possible transmission through the air. Feather dust may also carry the virus, providing more possibilities of transmission through air and through communal grooming. The virus may be transmitted through the egg as well, as ABV has been reported in parrot and duck eggs. Important research questions remain about transmission and why some birds become infected while others do not, even if exposed in the same manner.
Where did ABV in parrots come from?
PDD was first described in the late 1970s in young macaws imported from the Santa Cruz area of Bolivia. The great lethality of the disease to macaws and other large psittacines suggests it was a new disease in these species. So far, at least 80 other avian species have been shown to be infected with different strains of bornavirus. Most of these have been waterfowl. In waterfowl in North America, the most common strain is the goose genotype (ABV-CG), not a parrot strain.
It is hypothesized that borna viruses, like avian influenzas, may be a predominately waterfowl viruses that occasionally jump to other groups. One such jump may be responsible for the development of parrot ABV. At the time of the first documentation of PDD, many species of birds captured in the wild were imported into Europe and the USA. These birds were quarantined while being tested for Newcastle disease, and facilities were not designed to prevent cross-species contact.
Mixing of species, inadequate diet and high stress levels are factors that may support enhanced viral transfer, and may have facilitated development of disease in parrots, especially in young birds with developing immune systems. The presence of multiple different ABV genotypes in psittacine birds suggests there were multiple introductions, perhaps from different sources, or mutations of the virus within the parrots. To date there has been relatively little evaluation of other wild birds for the presence of different ABV genotypes; thus, the potential for discovering other genotypes in other species and geographic locations is high.
Supporting Information
For more information and updates to current research and treatment, check out:
- Hoppes, S.M. & Shivaprasad, H.L. Update on Avian Bornavirus and Proventricular Dilatation Disease: Diagnostics, Pathology, Prevalence, and Control. (2020) Vet Clin North Am Exot Anim Pract. 23(2):337-351.
- Boatright-Horowitz, S. L. 2020. Avian Bornaviral Ganglioneuritis: Current Debates and Unanswered Questions. Vet Med Int 2020:6563723.
- Tizard, I., Shivaprasad, H.L., Guo, J., Hameed, S., Ball, J. & Payne, S. The pathogenesis of proventricular dilatation disease (2016). Anim Health Res Rev. 17(2):110-126.
Supporting Literature
- Fluck, A., Enderline, D., Piepenbring, A., Heffels-Redmann, U., Herzog, S., Pieper, K., Herden, C. & Lierz, M. Correlation of avian bornavirus-specific antibodies and viral ribonucleic acid shedding with neurological signs and feather-damaging behavior in psittacine birds. (2019) Vet. Rec. 184(15):476.
- Rossi G, Crosta L, Ceccherelli R, Pesaro S. New evidence in PDD pathogenesis: can ganglioside sensitization satisfy Koch’s postulates? (2009) Proc Eur Assoc Avian Vet. Antwerp, Belgium;155.
- Hoppes S, Heatley J, Guo J, et al. Meloxicam treatment in cockatiels (Nymphicus hollandicus) infected with avian bornavirus. (2013) J Exotic Pet Med 22:275–279.
- Escandon P., Heatley, J., Tizard, I., Guo, J., Shivaprasad, H. & Musser, J. treatment with nonsteroidal anti-inflammatory drugs fails to ameliorate pathology in cockatiels experimentally infected with parrot bornavirus-2 (2019) Vet Med (Auckl) 10:185-195
- Reuter, A., Horie, M., Hoper, D., Ohnemus, A., Narr, A., Rinder, M., Beer, M., Staeheli, P., Rubbenstroth, D. Synergistic antiviral activity of ribavirin and interferon-a against parrot bornaviruses in avian cells. (2016). J. Gen Virol. 97(9): 2096-2103.
- Tokunaga, T., Yamamoto, Y., Sakai, M., Tomonaga, K. & Honda, T. Antivral activity of favipiravir (T-705) against mammalian and avian bornaviruses (2017). Antiviral Res. 143:237-245
- Rossi, G., Dahlhausen, R.D., Galosi, L., & Orosz, S.E. Avian Ganglioneuritis in clinical practice (2018). Vet Clin Exot Anim 21(1): 33-67.
- Matsumoto, Y, Hayashi, Y, Omori, H, Honda, T, Daito, T, Horie, M, Ikuta, K, Fujino, K, Nakamura, S, Schneider, U, Chase, G, Yoshimori, T, Schwemmle, M and Tomonaga, K. Bornavirus closely associates and segregates with host chromosomes to ensure persistent intranuclear infection. (2012) Cell Host & Microbe 11: 492–503.
- Stitz, L, Schilken, D and Frese, K. Atypical dissemination of the highly neurotropic Borna disease virus during persistent infection in cyclosporine A-treated, immunosuppressed rats. (1991) J. Virol. 65: 457–460.
- Monaco E, Hoppes S, Guo J, Tizard I. The detection of avian bornavirus within psittacine eggs. (2012) J Avian Med Surg. 26(3):144-8.
- Payne SL, Delnatte D, Guo J, Heatley JJ, Tizard I and Smith DA. Birds and Bornavirus. (2012). Animal Health Res. Rev. 13: 145–156.
- Hoppes S, Gray PL, Payne S, et al. The isolation, pathogenesis, diagnosis, trans- mission, and control of avian bornavirus and proventricular dilatation disease. (2010). Veterinary Clin North Am Exot Anim Pract 13(3):495–508.
- Leal de Araujo J, Hameed SS, Tizard I, Escandon P, Giaretta PR, Heatley JJ, Hoppes S, Rech RR. Cardiac Lesions of Natural and Experimental Infection by Parrot Bornaviruses. (2020) J Comp Pathol. 174:104-112.
- Boatright-Horowitz SL. Avian Bornaviral Ganglioneuritis: current debates and unanswered questions. (2020) Vet. Med. Int.
- Wuest, E., Malberg, S., Petzold, J., Enderlein, D., Heffels-Redmann, U., Herzog, S., Herden C., & Lierz, M. (2022) Experimental infection of embryonic cells and embryonated eggs of cockatiels (Nymphicus hollandicus) with two parrot bornavirus isolates (PaBV-4 and PaBV-2). Viruses 14(9):19884
