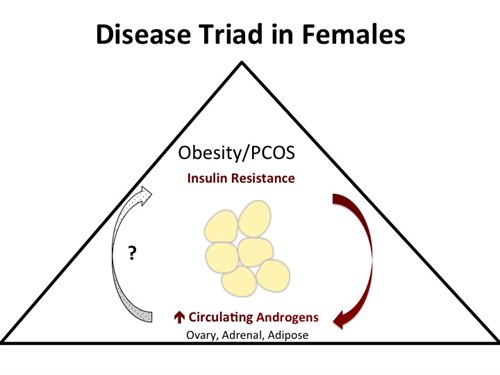
The hallmark of polycystic ovary syndrome (PCOS) is high circulating androgen concentrations or hyperandrogenemia. Hyperandrogenemia coupled with a high fat, western style diet puts PCOS patients at risk for obesity and metabolic syndrome. In addition to abberrant steroidogenic enzyme function in PCOS patients, hyperinsulinemia may drive excessive production of androgens by the adrenal, ovary, and possibly the adipose tissue. Evidence suggests that insulin resistance is higher in women whose white adipose tissue distribution is predominantly visceral. Interestingly, the role of androgens in glucose homeostasis is sexually dimorphic, with insulin resistance occurring in hyperandrogenemic females but in hypoandrogenemic males. In fact, sex specific effects of androgens and their interaction with other sex steroids could explain differential body fat deposition and insulin sensitivity between females and males.
Preliminary data generated in our laboratory in lean pigs indicates that testosterone may cause hyperinsulinemia systemically in addition to resulting in abnormal insulin signaling in adipose tissue, particularly the visceral adipose tissue compartment. Subsequent in vitro experiments performed in our laboraotry with induced, cultured rat adipocytes have demonstrated that female rat adipocytes collected during proestrus (i.e. high estradiol) are more sensitive than male rat adipocytes to the effects of androgens on the insulin signaling cascade at the gene transcript level. Adipocytes derived from the visceral adipose tissue compartment in female rats respond to the potent androgen, dihydrotestosterone, with down regulation of insuling signaling cascade components at the gene transcript level and decreased abundance of phosphorylated, activated proteins in the insulin signaling cascade. By contrast, adipocytes derived from the visceral adipose tissue compartment in male rats show no significant response in insulin signaling cascade proteins to androgen treatment.
The short-term goal of this portion of our research program is to establish proof of concept that androgens down-regulate distinct insulin-signaling pathway components in visceral versus subcutaneous adipose tissue of females and that this response is differentially affected by estradiol and progesterone and the development of obesity.