Help us determine if our new probiotic can help dogs and cats
Background and purpose:
Our pets’ intestines harbor a complex ecosystem consisting of millions of microorganisms. When the population of microorganisms in our pet’s gut is disturbed, we call it dysbiosis. Intestinal dysbiosis is commonly observed after antibiotic treatment and in pets presenting gastrointestinal disorders, for example, chronic enteropathies (Sung et al. 2022; AlShawaqfeh et al. 2017; Pilla et al. 2020; Werner et al. 2023). Our probiotic aims to help pets reestablish and maintain healthy intestines by providing a unique bacterium involved directly in the maintenance of intestinal health: Peptacetobacter hiranonis (Félix, Souza, and de Oliveira 2022; Correa Lopes et al. 2024; Sung et al. 2022; Blake et al. 2020).
Figure 1. Reduction of P. hiranonis (in red) and increased Dysbiosis Index (DI>2) were observed in dogs (A) and cats (B) with chronic enteropathies.
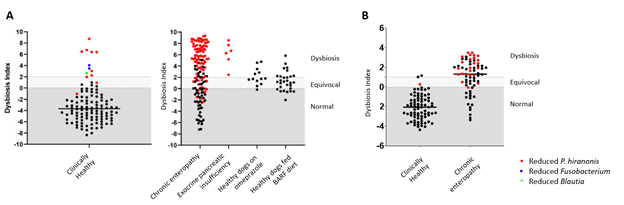
Dogs and cats have been sharing their lives with us, one of our aims at the Gastrointestinal Laboratory is to ensure that they have a healthy and happy life. We are dedicated to finding an answer on how to help our pets with intestinal dysbiosis, and our probiotic is a result of over ten years of microbiome research (J. S. Suchodolski 2016; Jan S Suchodolski 2022). By enrolling in our study, you are helping science advance and will receive free microbiome testing (Canine or Feline Dysbiosis Index) to know whether your dog or cat has intestinal dysbiosis.
What happens in this study?
If you are interested in having your pet participate in our study, please answer our questionnaire: https://docs.google.com/forms/d/e/1FAIpQLSdgjQqQWEAXwPPHoNz9yDLMkkVeLsXQNn9bno82mI1ReCEzZQ/viewform?usp=sharing to nominate your dog or cat for enrollment. All necessary materials, including probiotic treatment, sample collection kits, and instructions will be mailed to your home at no cost.
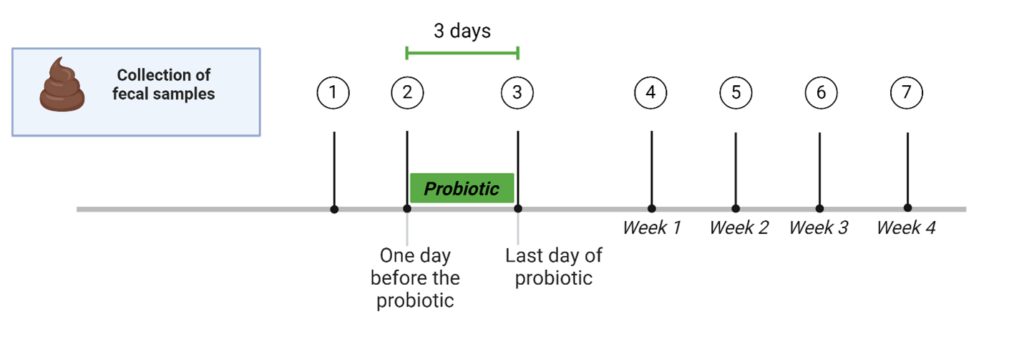
The probiotic treatment comprises a total of three pills (one pill per day) administered to your pet during their meals for three days. In total, we will need seven fecal samples from your pet:
Created with BioRender.com
Sample 1: Baseline fecal sample for screening
Sample 2: Fecal sample before probiotic
Sample 3: Fecal sample after probiotic
Sample 4: Fecal sample one week after probiotic
Sample 5: Fecal sample two weeks after probiotic
Sample 6: Fecal sample three weeks after probiotic
Sample 7: Fecal sample four weeks after probiotic
Pet owner responsibilities
Pet parents will be responsible for collecting samples and storing them properly until shipment. Our sample collection kit with samples can be dropped off at any FedEx location. Pet parents will need to follow the schedule for probiotic administration and fecal sample collection and answer a one-minute-long questionnaire for each fecal sample collected.
Participants requirements
This study will enroll healthy dogs and cats of any sex and breed and living within the United States.
Benefits and risks of participating
Possible but rare discomforts of probiotic administration may include nausea, vomiting, and diarrhea. Since our probiotic strain is a normal inhabitant of the canine and feline gastrointestinal tract, anticipated side effects on the animal are minimal. The collection of naturally voided fecal samples poses no discomfort or risks to the patients enrolled in the study.
If at study completion your dog or cat is still dysbiotic, we will offer additional standard treatment (fecal microbiota transplantation) free of charge.
Compensation
Upon enrollment, all costs related to this probiotic trial will be covered, including fecal sample collection, shipment of samples, probiotics, and the costs of the laboratory analysis of the fecal sample (i.e., Canine or Feline Dysbiosis Index).
Study duration and period
Enrollment in this clinical trial will last approximately six weeks, and it is our hope that owners who enroll in our project are dedicated to continuing enrollment for the entire six weeks. During this period, there will be seven fecal samples collected and shipped to the Texas A&M Veterinary Medical Teaching Hospital and owners will be required to provide the probiotic treatment to their pet and submit a response to the one-minute-long questionnaire for each one of the samples collected.
Recruitment period
From July 1st, 2024 to November 30th, 2024
Location
Texas A&M Small Animal Teaching Hospital
408 Raymond Stotzer Parkway, College Station, TX 77843
Contact
Feel free to contact Bruna Correa Lopes if you have any questions:
Dr. Bruna Correa Lopes brunalopes@tamu.edu
DVM | Graduate Research Assistant, Ph.D. student in Biomedical Sciences
Gastrointestinal Lab | Department of Small Animal Clinical Sciences
College of Veterinary Medicine | Texas A&M University
References:
For more information about Cat & Dog Microbiome Research: Dr. Jan S. Suchodolski – Gastrointestinal Laboratory (tamu.edu)
AlShawaqfeh, M. K., B. Wajid, Y. Minamoto, M. Markel, J. A. Lidbury, J. M. Steiner, E. Serpedin, and J. S. Suchodolski. 2017. “A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy.” FEMS Microbiol Ecol 93 (11). https://doi.org/10.1093/femsec/fix136. https://www.ncbi.nlm.nih.gov/pubmed/29040443
AlShawaqfeh, M. K., B. Wajid, Y. Minamoto, M. Markel, J. A. Lidbury, J. M. Steiner, E. Serpedin, and J. S. Suchodolski. 2017. “A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy.” FEMS Microbiol Ecol 93 (11). https://doi.org/10.1093/femsec/fix136. https://www.ncbi.nlm.nih.gov/pubmed/29040443
Blake, A. B., A. Cigarroa, H. L. Klein, M. R. Khattab, T. Keating, P. Van De Coevering, J. A. Lidbury, J. M. Steiner, and J. S. Suchodolski. 2020. “Developmental stages in microbiota, bile acids, and clostridial species in healthy puppies.” J Vet Intern Med 34 (6): 2345-2356. https://doi.org/10.1111/jvim.15928. https://www.ncbi.nlm.nih.gov/pubmed/33047396.
Correa Lopes, Bruna, Chih-Chun Chen, Chi-Hsuan Sung, Patricia Eri Ishii, Luis Fernando da Costa Medina, Frederic P Gaschen, Jan S Suchodolski, and Rachel Pilla. 2024. “Correlation between Peptacetobacter hiranonis, the baiCD Gene, and Secondary Bile Acids in Dogs.” Animals 14 (2): 216.
Félix, Ananda Portella, Camilla Mariane Menezes Souza, and Simone Gisele de Oliveira. 2022. “Biomarkers of gastrointestinal functionality in dogs: A systematic review and meta-analysis.” Animal Feed Science and Technology 283. https://doi.org/10.1016/j.anifeedsci.2021.115183.
Pilla, R., F. P. Gaschen, J. W. Barr, E. Olson, J. Honneffer, B. C. Guard, A. B. Blake, D. Villanueva, M. R. Khattab, M. K. AlShawaqfeh, J. A. Lidbury, J. M. Steiner, and J. S. Suchodolski. 2020. “Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs.” J Vet Intern Med 34 (5): 1853-1866. https://doi.org/10.1111/jvim.15871. https://www.ncbi.nlm.nih.gov/pubmed/32856349.
Suchodolski, J. S. 2016. “Diagnosis and interpretation of intestinal dysbiosis in dogs and cats.” Vet J 215: 30-7. https://doi.org/10.1016/j.tvjl.2016.04.011. https://www.ncbi.nlm.nih.gov/pubmed/27160005.
Suchodolski, Jan S. 2022. “Analysis of the gut microbiome in dogs and cats.” Veterinary Clinical Pathology 50: 6-17.
Sung, C. H., S. Marsilio, B. Chow, K. A. Zornow, J. E. Slovak, R. Pilla, J. A. Lidbury, J. M. Steiner, S. Y. Park, M. P. Hong, S. L. Hill, and J. S. Suchodolski. 2022. “Dysbiosis index to evaluate the fecal microbiota in healthy cats and cats with chronic enteropathies.” J Feline Med Surg 24 (6): e1-e12. https://doi.org/10.1177/1098612X221077876. https://www.ncbi.nlm.nih.gov/pubmed/35266809.
Werner, M., P. E. Ishii, R. Pilla, J. A. Lidbury, J. M. Steiner, K. Busch-Hahn, S. Unterer, and J. S. Suchodolski. 2023. “Prevalence of Clostridioides difficile in Canine Feces and Its Association with Intestinal Dysbiosis.” Animals (Basel) 13 (15). https://doi.org/10.3390/ani13152441. https://www.ncbi.nlm.nih.gov/pubmed/37570250.

