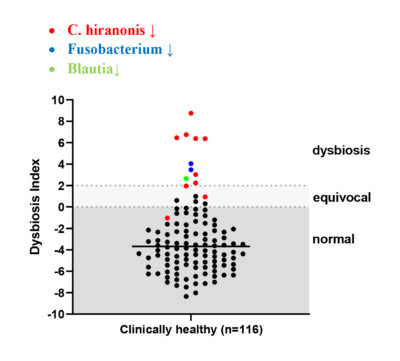
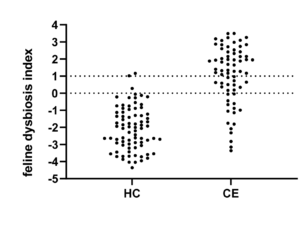
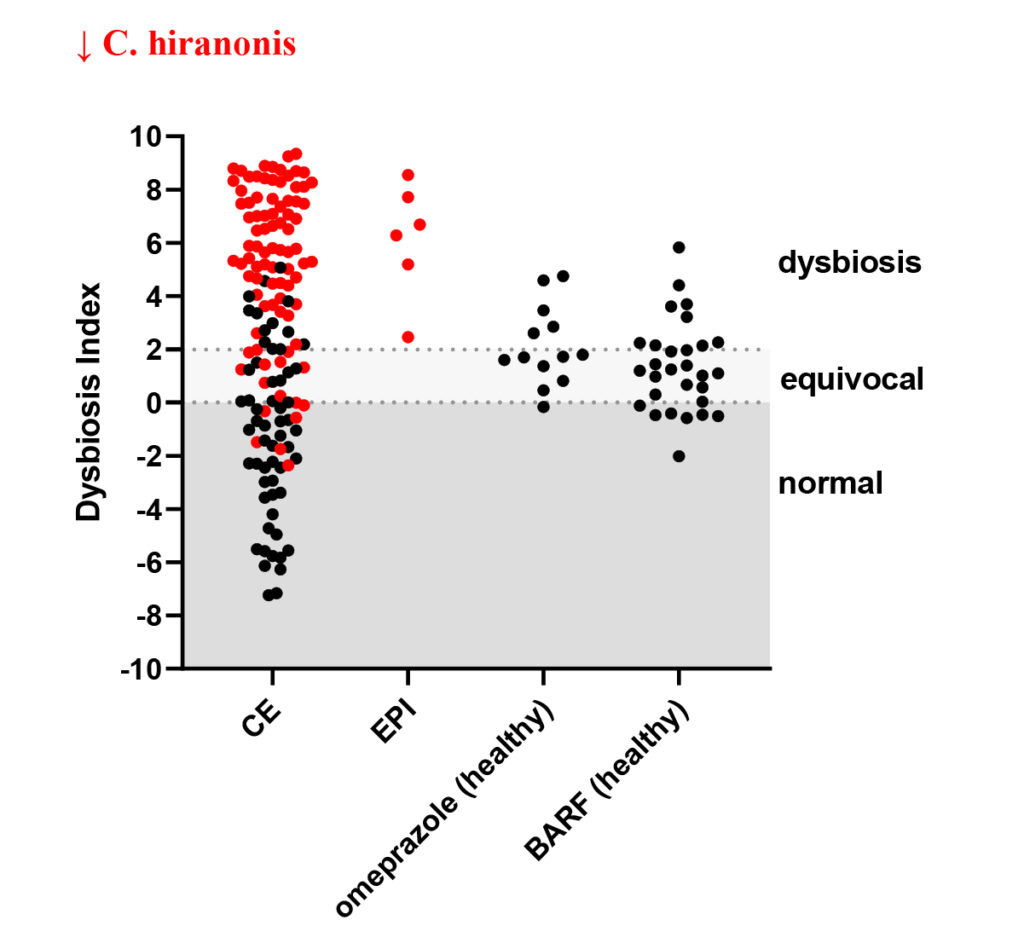
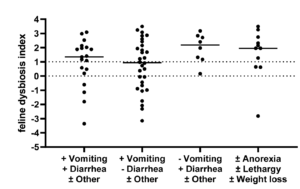
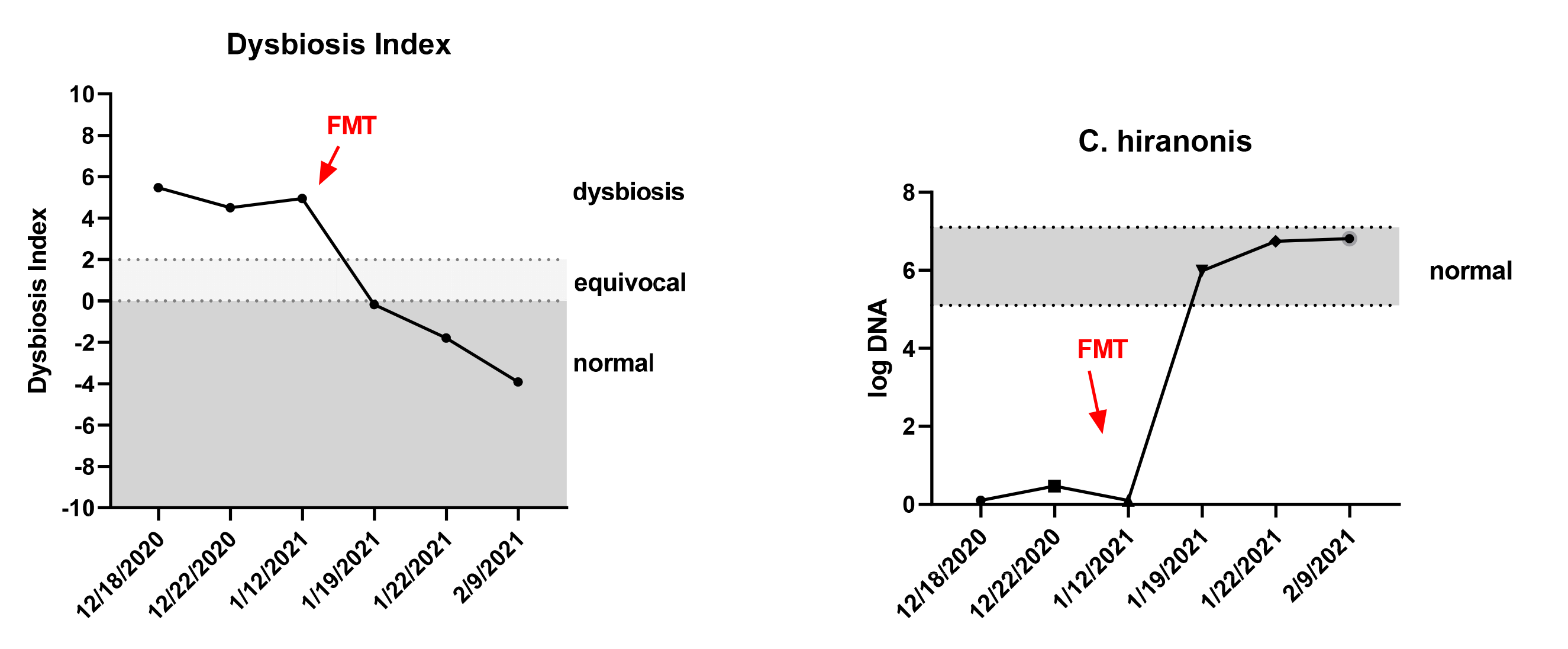
The dysbiosis index (DI) is a quantitative PCR-based assay that can be used to assess the feline (Sung et al, JFMS 2022) or canine (AlShawaqfeh M et al, FEMS 2017) fecal microbiome in individual patients. It is currently the only analytically validated assay to assess the fecal microbiome and has been used in various published clinical studies. The DI quantifies the fecal abundance of seven bacterial taxa as well as the total bacterial abundance. These bacterial taxa are commonly altered in chronic enteropathies (CE) and after broad-spectrum antibiotic use. The DI provides reference intervals for these bacterial groups and additionally calculates a single number that expresses the extent of intestinal dysbiosis (Table 1). The DI correlates negatively with species richness, i.e., a higher DI indicates lower microbial diversity.
The DI also predicts, by reporting the abundance of the bile acid converting bacterium Peptacetobacter (Clostridium) hiranonis, the ability of the intestinal microbiota to convert primary to secondary bile acids (BA). Normal amounts of secondary bile acids are antimicrobial and suppress potential enteropathogens, such as C. difficile, C. perfringens, and E. coli. Therefore, a decreased abundance of P. hiranonis and decreased conversion of primary to secondary BA strongly correlates with fecal dysbiosis. An increased DI together with decreased abundance of P. hiranonis is commonly seen in EPI and CE or after antibiotic administration.
| Table 1. Reference intervals for dogs and cats | ||||
| Function | normal in Dogs | normal in Cats | Change in dysbiosis | |
| Faecalibacterium | anti-inflammatory, production of SCFA | 3.4 – 8.0 | 3.8 – 8.4 | ↓ |
| Turicibacter | production of SCFA | 4.6 – 8.1 | 4.4 – 9.0 | ↓ |
| Blautia | production of SCFA | 9.5 – 11.0 | not measured | ↓ |
| Fusobacterium | production of SCFA | 7.0 – 10.3 | not measured | ↓ |
| Bifidobacterium | production of SCFA | not measured | 3.2 – 8.7 | ↓ |
| Bacteroides | production of SCFA | not measured | 4.0 – 7.5 | ↓ |
| Peptacetobacter (Clostridium) hiranonis | conversion of primary to secondary bile acids | 5.1 – 7.1 | 4.5 – 7.1 | ↓ |
| Streptococcus | overgrowth associated with dysbiosis | 1.9 – 8.0 | 1.6 – 5.2 | ↑ |
| E. coli | pro-inflammatory | 0.9 – 8.0 | 1.4 – 7.0 | ↑ |
| Dysbiosis Index (DI) | < 0 Normal DI indicating that no shifts in the overall diversity of the intestinal microbiota have been detected. If individual bacterial groups are outside the reference interval, this is suggestive of mild dysbiosis. |
< 0 Normal DI indicating that no shifts in the overall diversity of the intestinal microbiota have been detected. If individual bacterial groups are outside the reference interval, this is suggestive of mild dysbiosis. |
||
| 0 – 2 (DI) is mildly increased, suggesting a mild to moderate shift in the overall diversity of the intestinal microbiota |
0 – 1 (DI) is mildly increased, suggesting a mild to moderate shift in the overall diversity of the intestinal microbiota |
|||
| > 2 DI is significantly increased, consistent with a shift in the overall diversity of the intestinal microbiota |
> 1 DI is significantly increased, consistent with a shift in the overall diversity of the intestinal microbiota |
|||
| Data expressed log DNA/gram of feces | ||||
| SCFA = short-chain fatty acids | ||||