The Gastrointestinal Laboratory (GI lab) at Texas A&M University is pleased to offer a histopathology specialty service focused on gastrointestinal, hepatic, and pancreatic pathology. This service aims to provide high-quality interpretation of biopsy specimens by a team of pathologists with over a century of combined expertise in diagnostic pathology.
Notice:
We are now offering biopsy submission kits for purchase. Our goal is to help streamline the process of submitting to our histology lab, as well as ensure the integrity and quality of your specimens. These kits are available in two sizes. To purchase, please contact us via gilabhisto@tamu.edu.
60 mL kit – $76
This kit contains 24 jars that hold two biopsy cassettes each.
120 mL kit – $96
This kit contains 24 jars that hold four biopsy cassettes each.
Each kit includes biopsy cassettes, sponges, a pencil to label cassettes, and transport bags. Shipping costs of the kits are included in the price above. Please click here for Tissue Handling Guidelines.
Gastrointestinal pathology and sampling
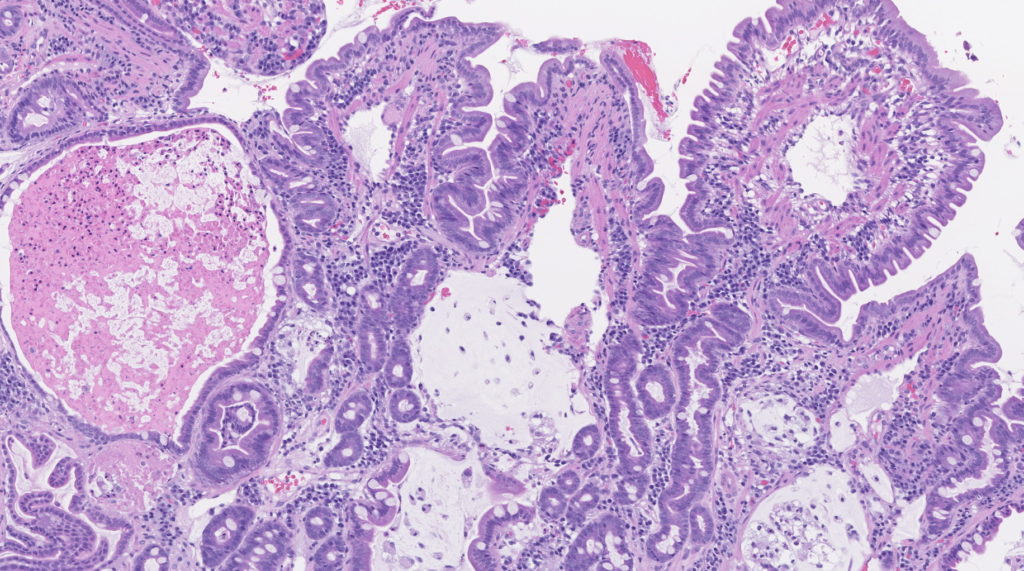
Histopathologic interpretation is often dependent on both the clinical history and specific questions being asked. Given the significant overlap in histopathologic patterns between different clinical syndromes, a complete clinical history, including results of laboratory testing, response to dietary trials, and gross findings during endoscopy/surgery is essential for proper interpretation. This information enables our pathologists to correlate histopathologic changes and clinical findings, providing relevant information that can be used to guide clinical decisions.
A completed histopathology submission form must be submitted with the samples. The histopathology submission form is available through the clinic portal. Additional clinical history can be attached to the submission form or emailed to gilabhisto@tamu.edu.
The selection of sampling technique, endoscopic or surgical, relies on correlation with the main clinical differentials and the overall status of the patient. The advantages of endoscopy are:
Endoscopy Pros
- Fast and minimally invasive;
- Allows gross examination of the mucosal surface; and
- Many samples can be obtained from each segment (10 to 20 in some cases).
Endoscopy Cons
- Cannot sample the jejunum, distal duodenum in large dogs, and occasionally visualizing or passing through the ileocolic sphincter to access the ileum in cats and small dogs can be difficult;
- Some lesions can be dense/hard leading to poor quality samples (e.g., pythiosis, scirrhous carcinomas);
- Sample quality can be variable;
- Lesions in the submucosa, muscularis, and serosa are not represented; and
- Patchy/segmental distribution of some conditions without changes elsewhere in the small intestine (e.g. Intestinal lymphoma in cats only affecting the jejunum without duodenal involvement).
Laparotomy/Laparoscopy Pros
- Allowing for any lesions to be sampled regardless of texture or location;
- Sampling of the submucosa, muscularis layers, and serosa;
- The ability to sample the jejunum in all patients and distal duodenum in large dogs;
- Ability to palpate the entirety of the small intestine; and
- Samples from other organs such as lymph nodes, liver, and pancreas can be obtained at the same time.
Laparotomy/Laparoscopy Cons
- Inability to visualize subtle mucosal changes that may not be noted from serosal examination;
- Full-thickness samples do not always equal excellent quality, as they can still be crushed or poorly oriented;
- A slight risk of biopsy site dehiscence;
- Greater post-procedure morbidity given the longer anesthesia and invasive nature of the procedure; and
- A limited number of samples are obtained.
Selection of sampling technique is often a clinical decision, and our pathologists are available to discuss options and strategies before sample acquisition. Further information regarding sampling techniques and possible caveats of gastrointestinal biopsies are available in these review papers:
Jergens AE, Willard MD, Allenspach K. Maximizing the diagnostic utility of endoscopic biopsy in dogs and cats with gastrointestinal disease. Vet J. 2016 Aug;214:50-60. DOI: 10.1016/j.tvjl.2016.04.008. Epub 2016 Apr 19. PMID: 27387727.
Mansell J, Willard MD. Biopsy of the gastrointestinal tract. Vet Clin North Am Small Anim Pract. 2003 Sep;33(5):1099-116. DOI: 10.1016/s0195-5616(03)00056-1. PMID: 14552163.
Liver sampling and histochemical stains
Wedge liver samples or laparoscopic cup biopsies are generally preferred as adequately sized samples can be acquired from multiple liver lobes. If it is not possible to obtain liver samples using these preferred techniques, ultrasound-guided needle hepatic biopsies may also be useful for diffuse hepatopathies but do have many limitations. For medium and large-breed dogs, a 14-gauge needle can be used, however, small dogs and cats require a 16-gauge needle. Submission of needle samples within cassettes with sponges can prevent fragmentation and improve diagnostic yield. It should be emphasized that needle biopsy is less preferable than surgical wedge or laparoscopic biopsy, as evaluation of hepatic architecture is limited given the size limitation.
Liver disease is often heterogeneous, and the severity or presence of histological changes can vary between lobes or areas. Furthermore, even if a focal lesion is noted, sampling of grossly normal liver may be beneficial. Usually, a minimum of 3 liver lobes should be sampled in addition to focal lesions. If lobes are grossly identical, samples may be submitted in a single jar and processed together. This enables histological evaluation and histochemical staining to be done more efficiently. Focal lesions should be submitted separately. It can also be helpful to provide the pathologist with images of lesions.
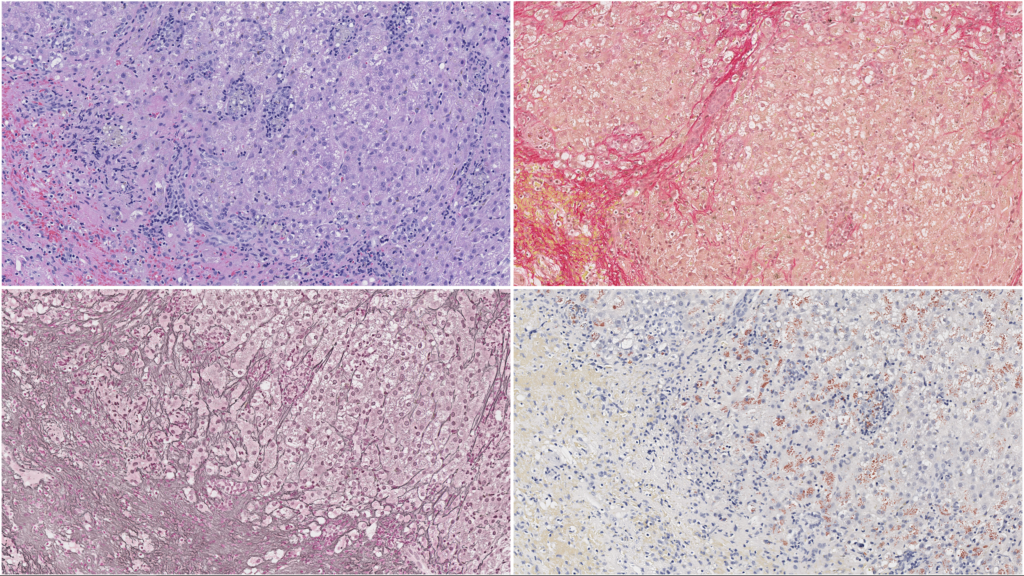
In addition to routine H&E-stained sections, a panel of special stains greatly enhances interpretation of liver samples. Connective tissue stains highlight the distribution and amount of fibrillar collagen in the liver. The collagen distribution also outlines the hepatic architecture and the distribution of lobular elements. A reticulin technique stains reticular fibers (mostly collagen type III), which assist in evaluating liver architecture, recognizing of proliferative foci, and defining areas of parenchymal collapse/extinction. A copper stain is essential in determining the presence and distribution of copper and may assist guidance of chelation therapy in combination with a quantitative measurement. While hemochromatosis is uncommon in domestic species, an iron stain is useful in mapping areas of previous hepatocellular damage and Kupffer cell iron sequestration, as well as assisting in the identification of other pigments (e.g., hemosiderin, lipofuscin, and bile). All of these stains are included in our standard liver panel.
Further information regarding sampling techniques and indications for liver biopsies in dogs and cats are available in this review paper:
Lidbury JA. Getting the Most Out of Liver Biopsy. Vet Clin North Am Small Anim Pract. 2017 May;47(3):569-583. DOI: 10.1016/j.cvsm.2016.11.007. Epub 2017 Jan 9. PMID: 28081862.
Immunohistochemistry, and fluorescent in-situ hybridization (FISH)
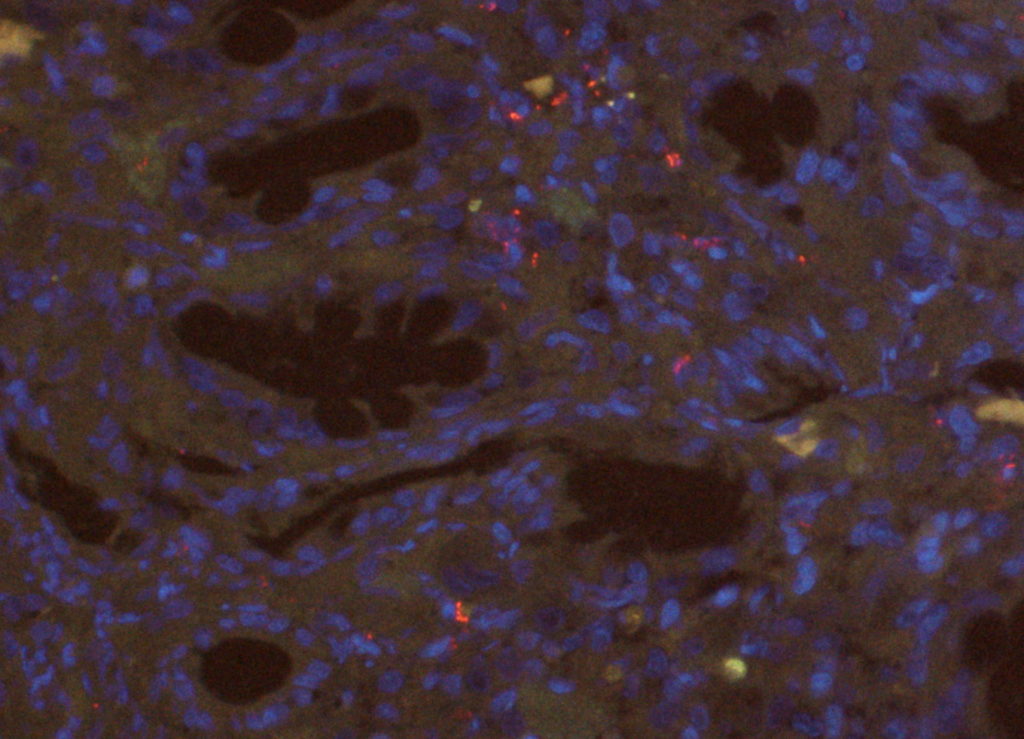
Our lab is prepared to offer most routine histochemical stains and immunohistochemistry. We will communicate which cases require ancillary testing and request authorization for any tests incurring additional fees.
Additionally, our lab has started offering in-house fluorescent in-situ hybridization (FISH) for the detection of bacteria. This technique enables us to identify bacteria in formalin-fixed paraffin-embedded (FFPE) tissues. FISH has a higher sensitivity when compared to traditional histochemical Gram staining. It is particularly useful for inflamed or necrotic tissue, as those can lead to abundant background staining in histochemical stains. We currently offer two FISH probes, one generic probe for Eubacteria, and a specific Escherichia coli/Shigella probe. The latter is most useful for cases with a suspicion of E. coli-associated granulomatous colitis or for the detection of invasive E. coli strains. FISH can be performed in biopsy samples routinely fixed in formalin and embedded in paraffin. We accept tissue samples in formalin, paraffin block, or unstained sections on charged slides (please submit five slides).
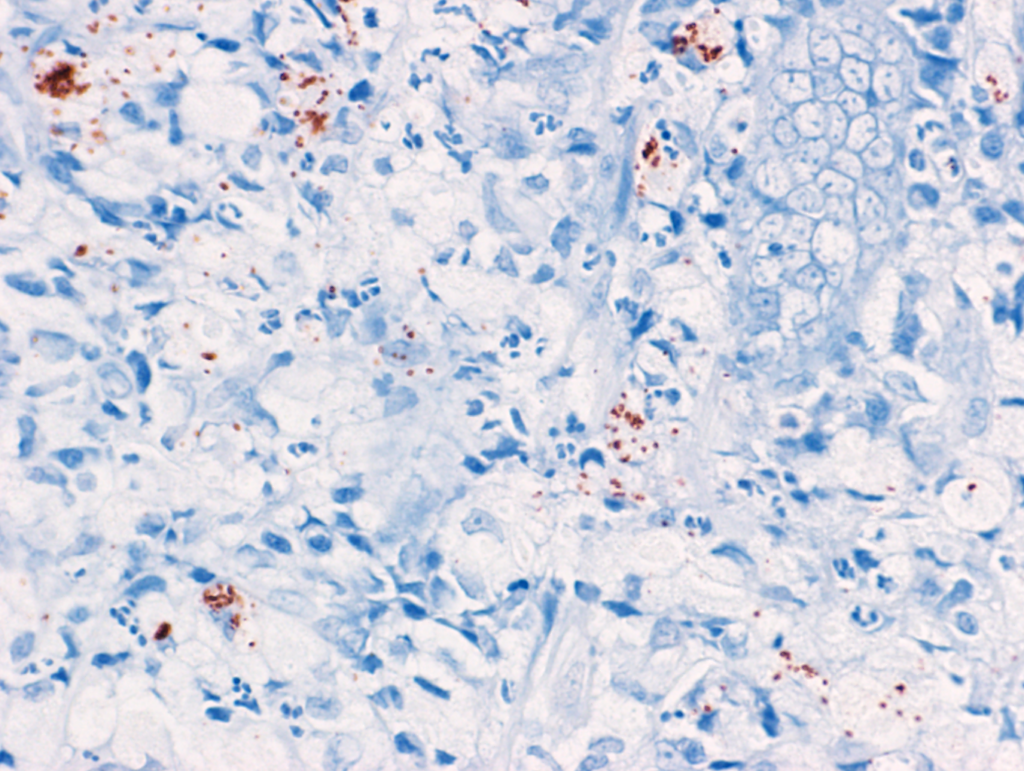
Immunohistochemistry (IHC) has also been proven as a sensitive method for the detection of invasive E. coli in dogs with E. coli-associated granulomatous colitis (histiocytic and ulcerative colitis of Boxer and French bulldogs) (Ishii et al., 2022). The advantages of this test are higher flexibility in sample processing and a lower cost compared to FISH. This test should be used only in cases suspected of E. coli-associated granulomatous colitis where PAS-positive macrophages were identified in the mucosa. More information is available in the following paper:
Ishii PE, Suchodolski JS, Duarte R, Pereira ARC, Lidbury JA, Steiner JM, Giaretta PR. Detection of invasive Escherichia coli in dogs with granulomatous colitis using immunohistochemistry. J Vet Diagn Invest. 2022 Nov;34(6):990-994. doi: 10.1177/10406387221119712. PMID: 35993285.
Shipping requirements
Please follow these recommendations for submission of biopsy samples:
1. Biopsies should be submitted in 10% neutral buffered formalin at a 10 to 1 ratio (i.e. 10 times the volume of formalin compared to tissue). It is recommended that biopsies from different intestinal segments are properly labeled in separate cassettes or containers.
2. Plastic, leakproof (screw top preferred) sample containers should be shipped in a sealed plastic bag (i.e., Ziploc). Please use specimen transport bags with a document holder, or place paperwork in a separate sealed bag. In the event of a formalin spill, this will ensure submission forms and other paperwork stay dry.
3. We are offering discounted shipping by using preprinted shipping labels available through our web page (please contact the lab for more information). Please note that the GI Lab does not accept packages marked “Bill Receiver”.
Please note that there is NO Saturday delivery for the GI Lab at Texas A&M University. Packages will sit unattended until we return on Monday. Note: Due to delivery procedures at such a large university, packages sent with the US Postal Service can often be delayed or even lost within departments. Please DO NOT use the US Postal Service as a carrier.
4. Somewhere on the outside of the package, as well as on the shipping label, please mark “Exempt Animal Specimen.” This is a requirement of the Texas Department of Transportation.
5. Please include a copy of the submission form with clinical history inside the package with your sample. Preferably in a specimen transport bag that has an additional pocket to separate paperwork from samples, OR in a separate sealed plastic bag (i.e., Ziploc). A copy of the clinical history can also be submitted via email to gilabhisto@tamu.edu.
International Shipments
We cannot accept samples from exotic or agricultural species from outside the United States. This includes feline and canine exotic species. If you have specific questions, please contact us directly via email (gilabhisto@cvm.tamu.edu) BEFORE shipping your samples.
In addition to the guidelines listed above, please attach the following statement below on your letterhead in the shipping documents on the outside of the box. Also include a copy inside the box on official letterhead with a signature. “This shipment contains only canine/feline tissue samples (word as appropriate and include the number of samples) for diagnostic testing. These samples are not contagious or infectious and have not been derived from animals exposed to agents of agricultural concern. This shipment has no commercial value.”

