Research Collaboration Discovers Medical Use For Chewing Gum Sweetener
Story by Alyssa Schaechinger, Texas A&M College of Engineering
A sweetener commonly found in chewing gum can replace toxic additives in hydrogels used to diagnose and treat a variety of medical conditions.
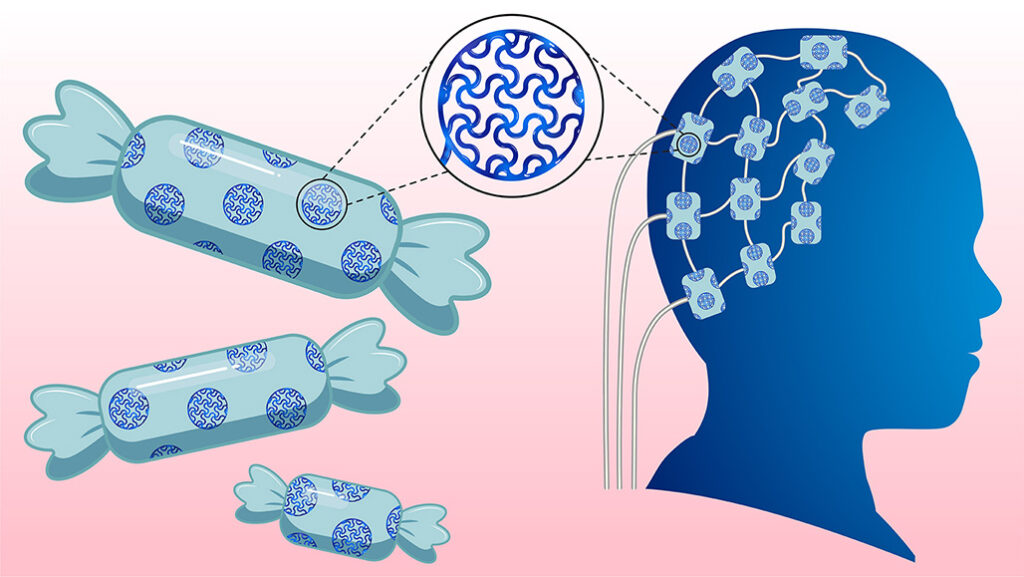
Imagine treating chronic illness not with pills, but with soft, flexible electronic implants seamlessly integrated into the body. The materials for such implants already exist — they just needed a sweet touch.
Electronic implants are commonly used to diagnose and treat various diseases and to restore lost motor and sensory functions. Conductive hydrogels increase an implant’s electrical conductivity and flexibility within the body, improving the overall effectiveness of electronic implants. However, traditional electrically conductive hydrogels contain toxic additives that may have negative impacts on patients after long-term use.
In a recent study published in Science Advances, researchers led by Dr. Limei Tian reported on a sweet solution to this problem: replacing these toxic additives with D-sorbitol, a safe sugar alternative commonly found in chewing gum.
“We’re excited by the potential to create bioelectronic devices that act like extensions of the body — soft, safe, and integrated with natural tissue,” said Tian, an associate professor in the Department of Biomedical Engineering and BMEN Excellence Fellow at Texas A&M University. “These devices could revolutionize treatments for neurological disorders, paralysis, and chronic pain, making long-term implants more viable and effective.”
The researchers used D-sorbitol to develop soft, stretchable hydrogels, which are better suited for the body than rigid materials. They can conform to delicate tissues like nerves and muscles, which reduces mechanical mismatch and lowers the risk of immune rejection.
This sweet new material can be used in a wide range of neural devices including brain implants for treating Parkinson’s disease and epilepsy and nerve interfaces to help restore movement in patients with spinal cord injuries. These hydrogels have the potential to be used in wearable biosensors for continuous health monitoring, electronic skin for prosthetics, and soft robotics with touch sensitivity.
“This biocompatible material makes electronic implants safer for the body and greatly enhances their electronic performance, paving the way for more reliable, long-term use in medical devices,” said Md Saifur Rahman, a Ph.D. student in Tian’s lab and a primary author of this work.
Challenges in creating a conductive hydrogel include biocompatibility and long-term stability. Many implants trigger adverse immune responses that lead to tissue scarring and device failure. The materials and devices must remain functional for years — ideally a lifetime — without degrading or harming surrounding tissue.
By replacing toxic additives with D-sorbitol, hydrogels will have increased biocompatibility due to lowered risk of negative immune responses and device rejection.
“Our goal was to create a fully biocompatible material, free of toxic additives, that outperforms traditional materials like platinum. And it did: our hydrogel electrodes demonstrated a higher capacity to store and deliver electrical charge than platinum, a key feature for effective neural stimulation,” said Tian.
The team tested their newly developed hydrogels on rats with successful results. The hydrogels exhibit mechanical and chemical properties comparable with biological tissues, reducing the risk of adverse immune reactions in patients. Prior to testing in humans, researchers plan to further refine the properties of hydrogels and evaluate their long-term stability in large animal models.
The research team plans to collaborate with clinicians and industry partners to translate this material into real-world medical devices. Their ultimate goal is to create next-generation neural interfaces that improve patient outcomes and push the boundaries of medical technology.
Dr. Feng Zhao, a professor in the Department of Biomedical Engineering and Dr. Hangue Park, an adjunct professor in the Department of Electrical and Computer Engineering collaborated on the project.
The study also included collaborators from Texas A&M’s College of Medicine and College of Veterinary Medicine & Biomedical Sciences (VMBS). Dr. Michelle Hook, an associate professor at the College of Medicine, and Dr. Yava Jones-Hall, a VMBS associate professor, further examined the hydrogels for their applicability to both human and veterinary medicine.
“I am a board-certified veterinary pathologist, and I analyzed the histological cross sections of nerves,” said Dr. Jones-Hall. “I discovered significantly more inflammation in the perineuronal tissue with implants containing platinum than there was surrounding nerves with electrically conductive hydrogel implants. These results supported Dr. Tian’s conclusions.”
###
For more information about the Texas A&M College of Veterinary Medicine & Biomedical Sciences, please visit our website at vetmed.tamu.edu or join us on Facebook, Instagram, and Twitter.
Contact Information: Jennifer Gauntt, Director of VMBS Communications, Texas A&M College of Veterinary Medicine & Biomedical Sciences, jgauntt@cvm.tamu.edu, 979-862-4216
You May Also Like








